Chemical Reactions
advertisement

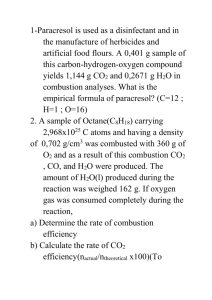
Three areas of focus Rearranging the building blocks of chemistry (atoms/ions/ molecules/electrons) Force & energy is what decides if the reaction occurs and how fast Mathematics helps you keep an inventory of all the starting and ending materials Recognize Evidence of a chemical change. Represent chemical reactions with equations. Change word equations into formula equations. Given a description of a reaction, write a word and formula equation. Balance chemical equations. Translate a formula equation into a sentence. Define and give a description of the major types of chemical reactions. Classify reactions as one of five major types. Predict the products of simple reactions when given the reactants. Understand, explain, and apply the activity series of the elements. Memorize the diatomic elements Memorize the symbols used in chemical equations. Use the Activity Series for single replacement reactions Use the Solubility Chart for Double Replacement Reactions Know common gases Memorize substances that decompose Carbonic acid, H2CO3 Sulfurous acid, H2SO3 Ammonium hydroxide, NH4OH NH3(g) H2CO3 (aq) H2O+ CO2(g) H2SO3(aq) H2O + SO2(g) NH4OH (aq H2O + The process by which one or more substances are rearranged to form different substances is called a chemical reaction. Also called a chemical change We are making something new! the reactants which enter into a reaction. the products which are formed by the reaction. the relative amounts of each substance used and each substance produced. Every chemical compound has a formula which cannot be altered. A chemical reaction must account for every atom that is used. This is an application of the Law of Conservation of Matter which states that in a chemical reaction atoms are neither created nor destroyed. Production of a Gas Temperature Change Color Change Production of a Solid (precipitate) Production of Water or other unionized substance chemical equations – represent reactions Reactants - starting Substances Products – ending substance Symbols?? The diatomic elements are always written H2, N2, O2, F2, Cl2, Br2, I2 The sign, → , means "yields" and shows the direction of the action. A small delta, ( ), above the arrow shows that heat has been added. A double arrow, ↔ , shows that the reaction is reversible and can go in both directions. Using words in equation form to represent a chemical reaction iron(s) + chlorine(g) iron(III) chloride(s) uses chemical formulas instead of words Fe (s) + Cl2 (g) ----- FeCl3 (s) Practice In order to obey the Law of Conservation of Mass equations must be Balanced: Coefficients: number written in front of a chemical formula to indicate the smallest number of particles involved in the reaction. Write skeleton equation. Change the coefficients to make the number of atoms of each element equal on both sides of the equation. NEVER CHANGE A SUBSCRIPT!!! Write the coefficients in the smallest ratio possible. Check your work. Start with “Big Formulas” C2H6O2 Save single elements for last O2 or Cu Balance hydrogens second to last Balance oxygens last Check for lowest ratio Do not change your subscripts Balance the polyatomic ions as one unit (if it didn’t break apart) Perform a final check If your equation doesn’t balance, check your formulas!! Five Types of Chemical Reactions Synthesis Reaction Decomposition Reaction Single Replacement Reaction Double replacement Reaction Combustion Reaction: oxygen combines with a substance and produces heat and light Synthesis Reaction: one product is formed from more than one simpler substances A + B AB Decomposition Reaction: One substance is broken down into one or more simpler substances: usually by the addition of energy AB A + B Single Replacement Reaction: atoms of one element replace another element in a compound A + BC B + AC Double replacement Reaction: involves the exchange of ions between two compounds AB + CD AD + CB Combustion Reaction: oxygen combines with a substance and produces heat and light X + O2 H2O + CO2 Ca + O2 CaO Br + LiI LiBr + I Al + Fe(NO3)2 Al(NO3)3 + Fe MgO + HCl MgCl2 + H2O C4H10 + O2 CO2 + H2O NH4NO2 NH3 + H2O (NH4)3PO4 + Sr(OH)2 Sr3(PO4)2 + NH4OH H2SO4 + NaOH Na2SO4 + H2O Zn + AgNO3 Zn(NO3)2 + Ag CuNO3 + KCl KNO3 + CuCl Given the reactants predict what is formed Write formulas for reactants Identify the type of reaction Rearrange the atoms to write formulas for products. Atoms of one element replace another element in a compound A + BC B + AC There are 3 Ways that a Single Replacement Reaction can occur. when zinc combines with iron (II) chloride the zinc replaces iron in the compound Z n + FeCl2 Fe + ZnCl2 Br2 + LiI LiBr + I2 Use the activity series of the elements If the free element is more active than the element in the compound the reaction will happen If the free element is below the element in the compound the reaction will not happen Bigger, stronger, orange shirted guy replaces white shirt guy in the dancing couple Now we have new couple and new single guy Some Examples to Observe before lab http://www.harpercollege.edu/tmps/chm/100/dgodambe/thedisk/se ries/3perform.htm two ionic compounds are mixed together in water In water the ionic compounds split into anions and cations. The cations have an opportunity to swap anions A reaction occurs, if by swapping anions, a product is formed that cannot split apart into anions and cations AB + CD AD + CB A reaction occurs when a pair of ions comes together to produce a substance that removes ions from the solution. one of the following must occur a precipitate: a solid produced during a reaction a gas Water or other unionized substance a product that decomposes http://www.wisconline.com/objects/ViewObject.aspx?ID=gch1404 What happens when one of the three possible products is not formed? Nothing All ions remain in solution (dissolved) NaNO3(aq) + KCl(aq)� � NaCl(aq) + KNO3(aq) Without a driving force there is no change in the solution so we say No Reaction has taken place Some double replacement reactions produce a gas. We observe this as bubbles or odors given off. Example: Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S(g) Some metathesis reactions do not give the product expected. the expected product (H2CO3) decomposes to give a gaseous product (CO2) CaCO3 (s) + HCl (aq) CaCl2 (aq) + H2CO3 CaCO3 (s) + HCl (aq) CaCl2 (aq) + CO2 (g) + H2O (l) Products that Decompose H2SO3 H2O + SO2 H2CO3 H2O + CO2 NH4OH H2O + NH3 These water molecules increase the number of solvent molecules and we see no observable evidence Usually accompanied by temperature change or Neutralization which can be seen with an acid base indicator Example: H2SO4 + NaOH Na2SO4 + H2O Generally, when solutions of an acid and a base are combined, the products are a salt and water HC2H3O2 (aq) + NaOH (aq) NaC2H3O2 (aq) + H2O (l) Acid + Base Salt + Water The molecular equation lists the reactants and products in their molecular form. AgNO3 (aq) + KCl (aq) AgCl (s) + KNO3 (aq) In the ionic equation all strong electrolytes (strong acids, strong bases, and soluble ionic salts) are dissociated into their ions. This more accurately reflects the species that are found in the reaction mixture. Ag+ (aq) + NO3- (aq) + K+ (aq) + Cl- (aq) To form the net ionic equation, cross out anything that does not change from the left side of the equation to the right. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) The only things left in the equation are those things that change (i.e., react) during the course of the reaction. Ag+(aq) + Cl-(aq) AgCl (s) Those things that didn’t change (and were deleted from the net ionic equation) are called spectator ions. Ag+(aq) + NO3-(aq) + K+(aq) + Cl-(aq) AgCl (s) + K+(aq) + NO3-(aq) Write a balanced molecular equation. Dissociate all strong electrolytes (strong acids, strong bases, and soluble ionic salts) Cross out anything that remains unchanged from the left side to the right side of the equation. Write the net ionic equation with the species that remain. Hydrocarbon + oxygen CO2 + H2O Hydrocarbon: A compound of hydrogen and carbon The phrase "To burn" means to add oxygen unless told otherwise. Complete Combustion: Hydrocarbon + oxygen CO2 + H2O Complete combustion means the higher oxidation number is attained. Incomplete Combustion: Hydrocarbon + oxygen CO + H2O Incomplete combustion means the lower oxidation number is attained. If oxygen is sufficient, the products are carbon dioxide and water vapor. If oxygen is low, carbon monoxide will be produced. automobile engine inside a closed garage or charcoal grill indoors. Hydrocarbon (CxHy) + O2(g) → CO2(g) + H2O(g) EX. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) EX. 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g) C3H8 + O2 --> CO2 + H2O propane 3 carbons = 3 carbon dioxide molecules 8 hydrogen atoms = four H2O molecules. balance the oxygen • This combustion of acetylene reaction is exothermic, and enough energy is released to melt metal. Used in welding. Why So Hot?? Triple bond -multiple bonds -short bond length Demo: Methane Snake Reaction CH4 + 2O2 CO2 + 2H2O A + B AB Elem/Cmpd + Elem/Cmpd Compound One Product Metal + oxygen → metal oxide Nonmetal + oxygen → metallic hydroxide Mg(OH)2(s) CO2(g) + H2O(l) → ; H2CO3(aq) 2 Na(s) + Cl2(g) → 2NaCl(s) acid salt A few nonmetals combine with each other MgO(s) + H2O(l) → Metal + nonmetal → CO2(g) nonmetallic oxide Nonmetallic oxide + water → C(s) + O2(g) → Metal oxide + water → 2Mg(s) + O2(g) → 2MgO(s) 2P(s) + 3Cl2(g) → 2PCl3(g) These two reactions must be remembered: N2(g) + 3H2(g) → 2NH3(g) NH3(g) + H2O(l) → NH4OH(aq) AB A + B Compound Cmpd/Elem + Elem/Cmpd One Reactant Metallic carbonates, when heated, form metallic oxides and CO2(g) Most metallic hydroxides, when heated, decompose into metallic oxides and water H2SO4 → H2O(l) + SO3(g) Some oxides, when heated, decompose 2KClO3(s) → 2KCl(s) + 3O2(g) Some acids, when heated, decompose into nonmetallic oxides and water Ca(OH)2(s) → CaO(s) + H2O(g) Metallic chlorates, when heated, decompose into metallic chlorides and oxygen CaCO3(s) → CaO(s) + CO2(g) 2HgO(s) → 2Hg(l) + O2(g) Some decomposition reactions are produced by electricity 2H2O(l) → 2H2(g) + O2(g) 2NaCl(l) → 2Na(s) + Cl2(g) A + B AB (synthesis) AB A + B (decomposition) A + BC B + AC (single replacement) AB + CD AC + BD (double replacement) Hydrocarbon + oxygen CO2 + H2O (combustion/oxidation)