Lipids
advertisement

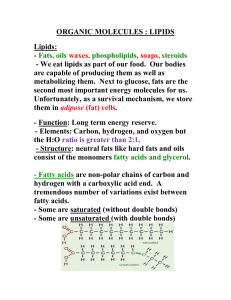
Lipids L. Scheffler IB Chemistry 1-2 Lincoln High School 1 Lipids • Lipids are organic molecules with long hydrocarbon chains that are soluble in non-polar organic solvents • Lipids are generally divided into three classes • 1.Triglycerides – Fats and Oils • 2. Phospholipids – lecithin • 3 Steroids -- Cholesterol 2 Fats and Oils • Fats and oils are triesters formed from the condensation reaction of glycerol (1,2,3,propanetriol) with long chain fatty acids • Example: 3 Fats and Oils • Fats are generally solids at room temperature, while oils are usually liquids • Fats contain saturated hydrocarbon chains • Oils contain unsaturated hydrocarbon chains, with at least 1 C=C. Frequently there are several C=C. They are known as polyunsaturated. 4 Fatty Acids • Stearic acid and linoleic acid have the same number of carbon atoms but very different melting points. 5 Common Fatty Acids Name Formula Source Saturated Fatty Acids Lauric Acid CH3-(CH2)10-COOH Coconut Oil Palmitic Acid CH3-(CH2)14-COOH Palm Oil Stearic Acid CH3-(CH2)16-COOH Animal and vegetable fats Arachidic Acid CH3-(CH2)18-COOH Peanut Oil Unsaturated Fatty Acids Oleic CH3-(CH2)7CH=CH-(CH2)7COOH Corn Oil Acid Linoleic CH3-(CH2)4 CH=CH-CH2-CH=CH – Linseed Oil Acid (CH2)7COOH 6 Differences in Melting Points • The carbon atoms in the hydrocarbon chain form a succession of tetrahedrons • This regular tetrahedral arrangement of carbon atoms makes it possible for it to pack with parallel chains fairly closely together • Although the attractions between the chains are only the rather weak van der Waals attractions the large surface area in the long carbon chains groups result in attractive forces that are strong enough to sustain a solid state 7 Unsaturated Fatty Acids • The presence of the C=C in the unsatruated fatty acid chain changes the bond angle from about 109 oC to around 120 oC. • This “kink” in the carbon chain keeps the fatty acids from packing as closely together. • As a result the van der Waals forces are weaker and less energy is required to separate them. 8 Saturated and Saturated Fats and Nutrition • Saturated and unsaturated fats are terms most commonly used in the context of nutrition. • Most animal fats are saturated fats. They are usually solids at room temperature. • Vegetable oils are more unsaturated. They are liquids at room temperature. • Oils with one C=C double bond per fatty acid chain are called “monounsaturated oils” • Oils with more than one C=C double bond per fatty acid chain are called “polyunsaturated oils”. 9 Hydrogenated Fats and Oils • Unsaturated oils can be hydrogenated to form solid, saturated fats by the reaction with hydrogen gas in the presence of nickel or platinum as a catalyst. • Margarine is an example of a hydrogenated oil. 10 The Iodine Index • The degree of unsaturation can be measured by measuring the amount of iodine that can react with the unsaturated fat or oil. Each mole of C=C requires one mole of I2 to react. • The haloalkane chain is nearly colorless Therefore unsaturated hydrocarbon chains will destroy purple brown color of iodine solutions as long as there are C=C bonds present. 11 Iodine Index of Common Fats/ Oils Oil or fat Butter fat Percent Percent of saturated monounsaturated fats fats 67% 29% Percent of Iodine polyunsaturated Index fats 4% 34 Beef Tallow 52% 44% 4% 50 Olive Oil 15% 75% 10% 81 Peanut Oil 18% 49% 33% 93 Canola Oil 7% 10% 62% 13% 31% 77% 130 125 Sunflower oil 12 Essential Fatty Acids • Most naturally occuring fats are a mixture of saturated, monounsaturated and polyunsaturated fatty acids • Essential fatty acids are those that the body cannot synthesize on its own. • They must be acquired from the foods we eat. 13 Essential Unsaturated Fatty Acids w-6 linoleic acid is an example of an essential fatty acid. It is a cis isomer. The w-6 indicates that there is a C=C on the 6th carbon from the end of the carbon chain 14 Essential Unsaturated Fatty Acids w-3 linolenic acid is another example of an essential fatty acid. It is a cis isomer. The w3 indicates that there is a C=C on the 3rd carbon from the end of the carbon chain 15 Trans Fatty Acids • When fatty acids are made synthetically by partially hydrogenating other polyunsaturated fatty acids, some trans isomers may be formed. • Trans fatty acids are found in fried foods and in some margarines. 16 Trans Fatty Acids • Trans fatty acids are generally considered undesirable since they increase the formation of LDL Cholesterol and hence the risk of heart disease. 17 Fat Metabolism • Fats metabolism occurs more slowly than carbohydrates metabolism but fats provide more energy than carbohydrates. • Fats require greater degree of oxidation to become CO2 and H2O than carbohydrates because carbohydrates already have one oxygen for every carbon atom • The number of oxygen molecules needed to oxidize a fat is greater than for carbohydrates., The oxidation of fats takes longer, but it also generates more energy. 18 Hydrolysis of Fats • In the body triglycerides, fats and oils, are hydrolyzed to fatty acids by the action of enzymes known as lipases 19 Hydrolysis of Fats 20 Phospholipids • A phospholipid has one of the three carbon chains of a triglyceride is replaced with a more polar phosphate-containing group. Four parts of a phospholipid 21 Phospholipids • In a phospholipids are one of the essential components of cell membranes. • Phosphatidyl Choline, an example of a phospholipid, has this structure: • Phosphatidyl choline is a major component of lecithin found in egg yolk, 22 Phospholipid Functions • Phospholipids form a significant part of cell membranes. • The cell membrane must protect the cell form the intercellular fluids around it. At the same time it must allow cell nutrients to enter the cell and waste products to leave. • Phospholipids tend to form bilayers in aqueous solutions. 23 Phospholipid Functions • The polar heads interface with water and the non-polar tails are attracted to nonpolar tissues. • The larger phospholipids can open and close to form vesicles. It is believed that this behavior is important to the functioning of the porous cell membranes 24 Cholesterol • Cholesterol has the characteristic four ring structure that is common to all steroids. • Cholesterol exists in esterified form in fatty acids and in a free form. • Cholesterol is created by the liver, but is also available through food. 25 Cholesterol Functions • Cholesterol is the most common, important and necessary steroid in the human body. • It is component of all tissues and is found in the blood, brain and the spinal cord. • It also acts as a building block to create other steroids such as sex hormones and adrenocorticoid hormones as well as essential substances such as vitamin D. 26 LDL and HDL • Cholesterol is transported around the body by lipoproteins. • Low density lipoproteins (LDL) range from 18-25nm • LDL transport cholesterol to the arteries where it can build up and cause cardiovascular disease • LDL result from saturated fats, especially lauric (C12), myristic (C14) and palmitic (C16) acids. • High density lipoproteins (HDL) are smaller, ranging from 8-11 nm. • HDL can remove cholesterol from the arteries and transport it back to the liver. 27 Lipid Functions in the Body • Energy storage Lipids are highly efficient energy stores for most higher animals. Fats are stored in the adipose tissues. Because they have less oxygen per molecule, lipids are oxidized more slowly, but release more energy. • Thermal insulation and protection Fats provide thermal insulation for the body. • Cell Structure Lipids, especially phospholipids, form a significant part of most cell membranes. They protect the cell from the intercellular fluids around it and play an important role in the transport of fluids into and out of the cell. 28 The end 29