3 rd LAW
advertisement
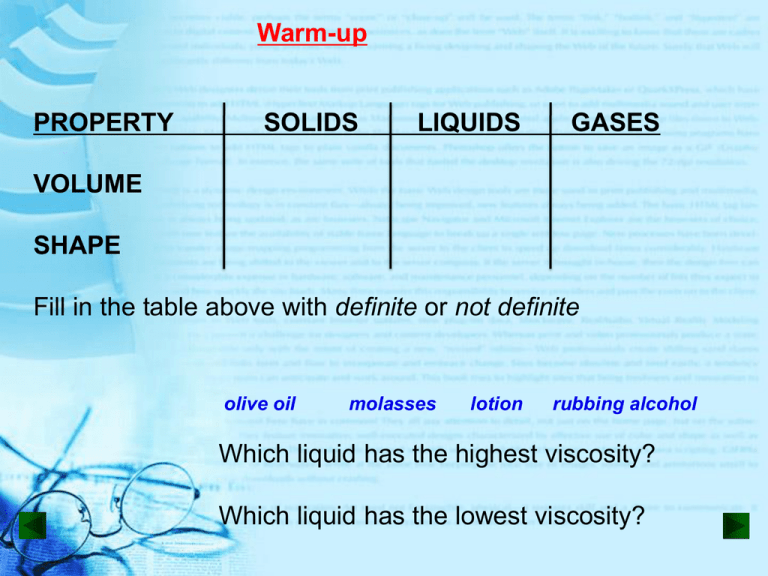
Warm-up PROPERTY SOLIDS LIQUIDS GASES VOLUME SHAPE Fill in the table above with definite or not definite olive oil molasses lotion rubbing alcohol Which liquid has the highest viscosity? Which liquid has the lowest viscosity? Section 15-2: Behavior of Gases Boyle’s, Charles’s rd and the 3 Law How full do you inflate a balloon for the Macy’s Thanksgiving Day Parade? The physical behavior of gas can be described by pressure (P), volume (V), and temperature (T) P, V, & T are interdependent (changing one, affects the others) •VOLUME *amount of space matter fills *measured in cm3, mL, L *volume of a gas, is the volume of its container •TEMPERATURE *measure of the average motion of the particles of a substance (remember - all particles move) *speedometer for particles *example: 20°C (room temp) is about 500 m/sec •TEMPERATURE *measured in Celsius, Kelvin, or Farenheit Low temperature: Cold particles, low energy, huddle to stay warm High Temperature: Hot particles, high energy, spread out •PRESSURE = FORCE AREA ( in units of kPa) *force of the outward push of the particles on their container Gas flows from areas of: High Pressure Low Pressure Questions: Why does an inflated ball leak when punctured? Why is a deflated balloon never completely flat? BOYLE’S LAW Robert Boyle (1627-1691) British scientist Boyle’s Law (squeeze a zit) T as P or T as P V V At a constant temperature, as the pressure increases the volume decreases, as the pressure decreases the volume increases Boyle’s Law: Varies Inversely Boyle’s Law What happens to helium balloons that are released and float to the heavens? Charles’s Law Jacques Alexandre César Charles (1746–1823), French scientist P as T or P as T V V Charles’s Law (Charlie leaves his flexible, basketball outside) At a constant pressure, as the temperature increases (particles move faster, collide more) the volume increases, as the temperature decreases the volume decreases Charles’s Law: Directly Proportional Charles’s Law If Charlie leaves his basketball outside, why is it deflated in the morning and fine by noon? rd 3 V as T or V as T LAW Rigid propane tank outside P P At a constant volume, as the temperature increases (particles move faster, collide more) the pressure increases, as the temperature decreases the pressure decreases 3rd Law 18-wheeler truck with rigid tires How full should a truck driver fill his tires in the morning, if he will be driving to Las Vegas, across the desert? Summary of Gas Laws Zit Boyles Law (inverse) •T P V Flexible Basketball Charles’s Law (direct) •P T V Rigid Propane 3rd Law (direct) •V T P