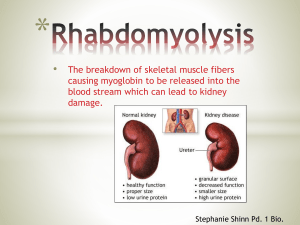
Clinical manifestations and diagnosis and treatment of rhabdomyolysis
advertisement

By Dr Sahar Karimpour Internal medicine resident Rhabdomyolysis is a syndrome characterized by muscle necrosis and the release of intracellular muscle constituents into the circulation. Rhabdomyolysis is characterized clinically by myalgias, red to brown urine due to myoglobinuria, and elevated serum muscle enzymes (including creatine kinase) . The degree of muscle pain and other symptoms varies widely. The characteristic triad of complaints in rhabdomyolysis is muscle pain, weakness, and dark urine . more than half of patients may not report muscular symptoms in contrast, occasional others may experience very severe pain. Muscle pain, when present, is typically most prominent in proximal muscle groups, such as the thighs and shoulders, and in the lower back and calves . Other muscle symptoms include stiffness and cramping. Additional symptoms that are more common in severely affected patients include malaise, fever, tachycardia, nausea and vomiting, and abdominal pain . Altered mental status may occur from the underlying etiology (eg, toxins, drugs, trauma, or electrolyte abnormalities). Muscle tenderness and swelling may be seen Muscle weakness may be present, depending upon the severity of muscle injury. Limb induration is occasionally present. Skin changes of ischemic tissue injury, such as discoloration or blisters, may also be seen but are present in less than 10 percent of patients The hallmark of rhabdomyolysis is an elevation in creatine kinase and other serum muscle enzymes. The other characteristic finding is the reddishbrown urine of myoglobinuria, but because this may be observed in only half of cases, its absence does not exclude the diagnosis. Serum creatine kinase (CK) levels at presentation are usually at least five times the upper limit of normal, but range from approximately 1500 to over 100,000 international units/L. The CK is generally of the MM or skeletal muscle fraction; a small proportion of the total CK may be from the MB or myocardial fraction. The presence of MB reflects the small amount found in skeletal muscle rather than the presence of myocardial disease. Elevations in serum aminotransferases are common and can cause confusion if attributed to liver disease. The serum CK begins to rise within 2 to 12 hours following the onset of muscle injury and reaches its maximum within 24 to 72 hours. A decline is usually seen within three to five days of cessation of muscle injury. CK has a serum half-life of about 1.5 days and declines at a relatively constant rate of about 40 to 50 percent of the previous day’s value. In patients whose CK does not decline as expected, continued muscle injury or the development of a compartment syndrome may be present. Myoglobin, a heme-containing respiratory protein, is released from damaged muscle in parallel with CK. Myoglobin is a monomer that is not significantly protein bound and is, rapidly excreted in the urine, often resulting in the production of red to brown urine. It appears in the urine when the plasma concentration exceeds 1.5 mg/dL Visible changes in the urine only occur once urine levels exceed from about 100 to 300 mg/dL, although it can be detected by the urine (orthotolidine) dipstick at concentrations of only 0.5 to 1 mg/dL . Myoglobin has a half-life of only two to three hours, much shorter than that of CK. Because of its rapid excretion and metabolism to bilirubin, serum levels may return to normal within six to eight hours. Both hemoglobin and myoglobin can be detected on the urine dipstick as “blood” microscopic evaluation of the urine generally shows few red blood cells (less than five per high-powered field) in patients with rhabdomyolysis whose positive test results from myoglobinuria . Such testing is not a reliable method for rapid detection of myoglobin if red blood cells are present or in patients with hemolysis due to its lack of specificity for myoglobin. Proteinuria may also be seen, due to the release of myoglobin and other proteins by the damaged myocytes . fluid and electrolyte abnormalities, many of which precede or occur in the absence of kidney failure, and hepatic injury cardiac dysrhythmias and risk of cardiac arrest may result from the severe hyperkalemia that occurs with significant myonecrosis . Later complications include acute kidney injury, compartment syndrome, and, rarely, disseminated intravascular coagulation. Hypovolemia results from “third-spacing” due to the influx of extracellular fluid into injured muscles and increases the risk of acute kidney injury Hyperkalemia and hyperphosphatemia result from the release of potassium and phosphorus from damaged muscle cells. Levels of potassium may increase rapidly, but the levels of potassium and phosphate decrease as they are excreted in the urine. Hyperkalemia is more common in patients with oliguric acute kidney injury Hypocalcemia, which can be extreme, occurs in the first few days because of entry into damaged myocytes and both deposition of calcium salts in damaged muscle and decreased bone responsiveness to parathyroid hormone. During the recovery phase, serum calcium levels return to normal and may rebound to significantly elevated levels due to the release of calcium from injured muscle, mild secondary hyperparathyroidism from the acute renal failure, and an increase in calcitriol (1,25dihydroxyvitamin D) Severe hyperuricemia may develop because of the release of purines from damaged muscle cells and, if acute kidney injury occurs, reduced urinary excretion. Metabolic acidosis is common, and an increased anion gap may be present. Acute kidney injury (AKI, acute renal failure) is a common complication of rhabdomyolysis. The reported frequency of AKI ranges from 15 to over 50 percent . The risk of AKI is lower in patients with CK levels at admission less than 15 to 20,000 units/L; risk factors for AKI in patients with lower values include dehydration, sepsis, and acidosis . Volume depletion resulting in renal ischemia, tubular obstruction due to heme pigment casts, and tubular injury from free chelatable iron all contribute to the development of renal dysfunction. Reddish-gold pigmented casts are often observed in the urine sediment. A compartment syndrome exists when increased pressure in a closed anatomic space threatens the viability of the muscles and nerves within the compartment . Compartment syndrome is a potential complication of severe rhabdomyolysis that may develop after fluid resuscitation, with worsening edema of the limb and muscle . Infrequently, severe rhabdomyolysis may be associated with the development of disseminated intravascular coagulation due to the release of thromboplastin and other prothrombotic substances from the damaged muscle . Indications for diagnostic testing: Both myalgias and pigmenturia Either myalgias or pigmenturia, with a history suggesting the presence or recent exposure to a potential cause or event The diagnosis should be suspected following prolonged immobilization, in any stuporous or comatose patient or in a patient who is otherwise unable to provide a medical history and has one or more of the following: Muscle tenderness Evidence of pressure necrosis of the skin Signs of multiple trauma or a crush injury Blood chemistry abnormalities suggesting the possibility of increased cell breakdown, such as hyperkalemia, hyperphosphatemia, and /or hypocalcemia Evidence of acute kidney injury Creatine kinase Urinalysis, including dipstick and microscopic evaluation Complete blood count, including differential and platelet count Blood urea nitrogen, creatinine, and routine electrolytes including potassium Calcium, phosphate, albumin, and uric acid Electrocardiography Additional testing, such as evaluation of suspected metabolic myopathy or toxicology screening for drugs of abuse, depends upon the clinical context. We make the diagnosis of rhabdomyolysis in a patient with either an acute neuromuscular illness or dark urine without other symptoms, plus a marked acute elevation in serum creatine kinase (CK). The CK is typically at least five times the upper limit of normal, and is usually greater than 5000 international units/L. No absolute cut-off value for CK elevation can be defined, and the CK should be considered in the clinical context of the history and examination findings Other muscle enzymes in addition to CK are typically elevated (eg, aldolase, aminotransferases, lactate dehydrogenase), but such testing is not usually necessary to make the diagnosis. In patients with dark urine, but without elevation in the CK, additional diagnostic considerations should be explored. A muscle biopsy is generally not required. Findings on biopsy include loss of normal crossstriations and cell nuclei and the absence of inflammatory cells . Electromyography (EMG) is generally not required. Findings are most often normal and are only mild when present. Magnetic resonance imaging (MRI) is generally not required. Findings in affected muscle include increased signal intensity on T2-weighted images and good contrast between normal and abnormal muscles on STIR images. There are recurrent episodes of rhabdomyolysis after exertion or in association with fasting or a viral illness. The last two associations occur most commonly with carnitine palmitoyltransferase deficiency and the other disorders of lipid metabolism. There is a history of exercise intolerance, recurrent cramps, and fatigue beginning in childhood, and episodes of pigmenturia occurring in adolescence. There is a family history of rhabdomyolysis or exercise intolerance, particularly in siblings, thereby suggestive of an autosomal recessive inheritance pattern. The individual has normal strength and muscle enzymes during interictal periods. One exception is muscle phosphorylase deficiency, a disorder in which chronic muscle weakness may develop after repeated episodes and CK levels do not return to normal between attacks Myocardial infarction Inflammatory myopathy Immune-mediated necrotizing myopathy Deep vein thrombosis Renal colic Recognition and management of fluid and electrolyte abnormalities, which should be initiated regardless of renal function and which may prevent severe metabolic disturbances and acute kidney injury. Identification of the specific causes and the use of appropriate countermeasures directed at the triggering events, including discontinuation of drugs or other toxins that may be etiologic factors Prompt recognition, evaluation, and treatment of compartment syndrome in patients in whom it is present. In addition to treating the underlying rhabdomyolysis or hemolysis, the general goals for preventive therapy in all patients at risk for heme pigment-induced AKI are twofold: Correction of volume depletion, if present Prevention of intratubular cast formation The goals of volume repletion are to maintain or enhance renal perfusion, minimizing ischemic injury, and to increase the urine flow rate which will limit intratubular cast formation, wash out partially obstructing intratubular casts, and increase urinary potassium excretion. Intravenous isotonic saline should be administered as soon as possible after the onset of injury or detection of hemolysis, and continued until the muscle injury or hemolysis has resolved. The optimal fluid and rate of repletion are unclear. In addition, the total amount and rate of volume repletion will vary depending on the underlying cause of rhabdomyolysis and hemolysis. For patients who are at risk for hemeassociated AKI due to rhabdomyolysis from any cause, we suggest initial fluid resuscitation with isotonic saline at a rate of 1 to 2 L/hour. The plasma creatinine kinase (CK) concentration correlates with the severity of muscle injury and concentrations >5000 U/L identify patients who are at risk for the development of AKI. All patients should be initially treated with vigorous fluid repletion until it is clear from sequential laboratory values that the plasma CK level is stable and not increasing. Patients who have a stable plasma CK level less than 5000 U/L do not require intravenous fluid since studies have shown that the risk of AKI is low among such patients . The initial rate is continued until the systemic blood pressure normalizes and urine output is established, or there is evidence of volume overload. If a diuresis is established, fluids are titrated to maintain a urine output of 200 to 300 mL/hour. Among patients with rhabdomyolysis, fluid repletion should be continued until plasma CK levels decrease to less than 5000 U/L and urine is dipstick negative for hematuria. Among patients with hemolysis, LDH and hemoglobin levels should be monitored to guide intravenous fluid therapy. A forced alkaline diuresis, in which the urine pH is raised to above 6.5, may diminish the renal toxicity of heme. In theory, urine alkalinization prevents hemeprotein precipitation with Tamm-Horsfall protein, and therefore intratubular pigment cast formation. Alkalinization may also decrease the release of free iron from myoglobin and the formation of F2isoprostanes, which may enhance renal vasoconstriction and decrease the risk for tubular precipitation of uric acid. In addition to a lack of clear evidence of benefit, maintaining the urine pH above 6.5 is difficult in patients with AKI. There are also potential risks to alkalinization of the plasma, such as promoting calcium phosphate deposition (which is more likely if hyperphosphatemia is present), and inducing or worsening the manifestations of hypocalcemia by both a direct membrane effect and a reduction in ionized calcium levels . Despite these limitations, patients who are appropriately monitored may benefit from bicarbonate therapy. We generally administer a bicarbonate infusion to patients who have severe rhabdomyolysis, such as those with a serum CK above 5000 U/L, or clinical evidence of severe muscle injury (eg, crush injury) and a rising serum CK, regardless of the initial value. In such patients, bicarbonate may be given, providing the following conditions are met: Severe hypocalcemia is not present Arterial pH is less than 7.50 Serum bicarbonate is less 30 meq/L We infuse 130 meq/L of sodium bicarbonate (150 mL [3 amps] of 8.4 percent sodium bicarbonate mixed with 1 L of 5 percent dextrose or water) via an intravenous line separate from that used for the isotonic saline infusion. The initial rate of infusion is 200 mL/hour; the rate is adjusted to achieve a urine pH of >6.5. If bicarbonate is given, the arterial pH and serum calcium should be monitored every two hours during the infusion. The bicarbonate infusion should be discontinued if the urine pH does not rise above 6.5 after three to four hours, if the patient develops symptomatic hypocalcemia, if the arterial pH exceeds 7.5 or if the serum bicarbonate exceeds 30 meq/L. If the bicarbonate solution is discontinued, volume repletion should be continued with isotonic saline. The benefit of loop diuretics or mannitol in rhabdomyolysis is not established. Experimental studies suggested that mannitol might be protective by causing a diuresis, which minimizes intratubular heme pigment deposition and cast formation, and/or by acting as a free radical scavenger, thereby minimizing cell injury. mannitol can lead to both volume depletion and, since free water is lost with mannitol, hypernatremia Mannitol administered in very high doses, or to patients with reduced renal excretion can cause hyperosmolality, volume expansion and hyperosmolar hyponatremia. The increase in plasma osmolality can also cause passive movement of potassium out of cells and raise the plasma potassium concentration. Acute renal failure may occur if patients are treated with more than 200 g of mannitol per day. The use of mannitol administration may be of benefit in patients with marked elevations in CK (>30,000 U/L), however, even in these patients with severe rhabdomyolysis, the true benefit associated with mannitol administration remains undefined. If mannitol is given, providing the urinary flow is adequate (defined as >20 mL/hour), adding 50 mL of 20 percent mannitol (1 to 2 g/kg per day [total, 120 g], given at a rate of 5 g per hour) to each liter of fluid is suggested. Mannitol is contraindicated in patients with oligoanuria. If mannitol is given, the plasma osmolal gap should be measured, and mannitol discontinued if the osmolal gap rises above 55 mosmol/kg Mannitol should be discontinued if the desired diuresis of approximately 200 to 300 mL/hour cannot be achieved, since there is a risk of hyperosmolality, volume overload, and hyperkalemia with continued mannitol administration under these conditions. Loop diuretics have no impact on outcome in AKI In the context of rhabdomyolysis, loop diuretics may worsen the already existing trend for hypocalcemia, since they induce calciuria and may increase the risk of cast formation judicious use of loop diuretics may be justified in patients with rhabdomyolysis or hemolysis if there is evidence of volume overload. Patients who remain oliguric or anuric despite aggressive volume resuscitation should be considered to have established AKI. Among such patients, the rate of fluid administration should be decreased to a rate sufficient to maintain circulatory support. Such patients should be closely followed for indications to initiate dialysis. The use of dialysis to remove myoglobin, hemoglobin or uric acid in order to prevent the development of renal injury has not been demonstrated . The initiation of dialysis may be necessary for control of volume overload, hyperkalemia, severe acidemia, and uremia in established AKI. Patients should be closely followed for the development of metabolic abnormalities including hyperkalemia, hypocalcemia, hyperphosphatemia, and hyperuricemia. To minimize the late occurrence of hypercalcemia, calcium supplementation for hypocalcemia should be avoided unless significant signs and symptoms of hypocalcemia develop or calcium administration is required for the management of hyperkalemia. Hyperkalemia should be anticipated and may occur even in the absence of severe renal failure. Hyperkalemia should be aggressively treated. Dialysis may be required to treat severe hyperkalemia. Patients who develop hyperuricemia should be treated with allopurinol . Allopurinol should be given orally at 300 mg if uric acid levels are >8 mg/dL (476 micromol/L) or if there is a 25 percent increase from baseline. Allopurinol is not indicated in the treatment of hemolysis in the absence of hyperuricemia. Other than maintenance of fluid and electrolyte balance and tissue perfusion, there is no specific therapy once the patient has developed AKI. The initiation of dialysis may be necessary for control of volume overload, hyperkalemia, severe acidemia, and uremia. Peritoneal dialysis may not be sufficient to achieve adequate metabolic control in patients with severe rhabdomyolysis, which may necessitate frequent hemodialysis or the use of high dose continuous renal replacement therapy Thanks for your kind attention