slides
advertisement
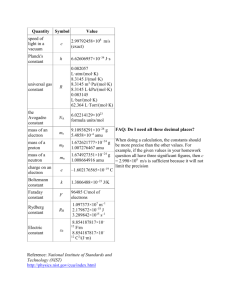
Unit 1: Stoichiometry and Reactions Sarah & Sara Nomenclature Cations—electron-deficient, (+) charge, charge is group # transition metals-charge shown with Roman Numeral in name exceptions: Zn2+ Ni2+ Ag+ the less charged cation of each atom, such as Copper uses the –ous ending, while the one with greater charge uses the –ic ending Copper (II) ion can also be called cuprous Nomenclature Anions—excess electrons, (-) charge, charge is # of columns from noble gases (**exceptions), monatomic –ide suffix polyatomic oxyanions –ate suffix most common (-ite with fewer O) oxyanions modified by H+ **cyanide = CN- hydroxide = OH- Nomenclature Neutral ionic compound = a salt = metal cation + nonmetal anion [empirical formula with net charge of zero] to name: modify cation and/or anion with subscripts [name the cation then the anion, the subscript is not stated, write transition metals with Roman Numerals for charge] Nomenclature Acids—have H+ cation, name is based on anion -ide = hydro_______ic acid -ate = ____________ic acid -ite = ____________ous acid EX: Chloride= hydrocholric acid Chlorate= chloric acid Chlorite= chlorous acid Nomenclature Covalent (or binary) compounds = 2 nonmetals (name farthest left first with subscripts stated in name, exceptions—Fluorine is always last, Oxygen is last unless with F) Organic compounds = hydrocarbons, only C and H; others can include O, N, and/or S alkanes – C “backbone,” all single bonds, as many H as necessary to fill bonds, end with –ane Name with # of C: 1=meth 2=eth 3=prop 4=but 5+ = binary prefixes alcohols – O-H “functional group” in place of 1 or more H [-ane becomes -anol] Number position in functional group in front (or to which C is it bonded) Nomenclature The –ates: Sulfate (SO4-) Chlorate (ClO3-) Phosphate (PO43-) Carbonate (CO32-) Nitrate (NO3-) Acetate (C2H3O2- = CH3COO-) Chromate (CrO42-) Dichromate (Cr2O72-) Permanganate (MnO4-) Hydride = HHydrogen ion = H+ ammonium ion = NH4+ Atomic Structure angstrom = 10-10 m = Å = size of atom in meters Weight of 1 electron = (1/1800) proton size of the electron cloud is the full atomic size and is about (1/4000) of an atom’s mass 1 x 10-10 to 5 x 10-10 m = 1 to 5 Å = .1 to .5 nm = 100 to 500 pm nucleus contains 99.9% mass of an atom; 1 x 10-15 to 1 x 10-14 m = .00001 to .0001 Å Isotope Notation A Z X q A = mass # = protons + neutrons Z = atomic # = protons q = protons – electrons Reactions and Other Stuff Decomposition reaction: Y C + D Synthesis/composition: A + B X Combustion: CxHyOz CO2 + H2O Avogadro’s Number = NA = 6.02 x 1023 Spectrometer average mass – weighted average Diatomic elements = H, O, N, Cl, Br, I, F Stoichiometry Stoichiometric Relationships: grams of A moles of A moles of B grams of B Limiting reactants: practical solutions (amounts of reactants are mismatched) all calculations are based on limiting reactant limiting reactant is completely consumed Method A: use one reactant to find how much of other reactant is needed fewer calculations, most efficient if only finding limiting reactant Method B: use both to find out how much product is made more calculations, fool proof strategic planning Yield! Theoretical Yield = grams of limiting reactant moles of limiting reactant moles of product grams of product % Yield=(actual or experimental) / theoretical Example Problem 1 C6H6 + Br2 C6H5Br +H2 You have 30.0g of C6H6 and 65.0g of Br2 present for a reaction. A) Find the limiting reactant, and state how much C6H5Br will be produced? B) Calculate the percent yield if 56.7g of C6H5Br are produced by the reaction. Solution to Example 1 A) First calculate the moles of each reactant you have present at the beginning of the reaction. 30.0g C6H6 x (1 mol C6H6 / 78.11g C6H6) = .384 mol C6H6 65.0g Br2 x (1 mol Br2 / 159.8g Br2) = .407 mol Br2 Because C6H6 and Br2 are in a 1:1 ratio, C6H6 is the limiting reactant and determines the theoretical yield. .384 mol C6H6 x (1 mol C6H5Br / 1 mol C6H6) x (157.0g C6H5Br / 1 mol C6H5Br) = 60.3g C6H5Br Solution fo Example 1 B) % yield = actual or experimental/ theoretical % yield = (56.7g C6H5Br actual / 60.3g C6H5Br theoretical) x 100 = 94.0% Example Problem 2 Calculate the percent composition of Carbon in each of the following molecules. A) C7H6O B )C8H8O3 C) C7H14O2 Solution to Example 2 A) C7H6O FW: 7(12.0 amu) + 6(1.0 amu) + 1(16.0 amu) = 106.0 amu %C = ( 7(12.0 amu) / 106.0 amu ) x 100 = 79.2% Solution to Example 2 B )C8H8O3 FW: 8(12.0 amu) + 8(1.0 amu) + 3(16.0 amu) = 152.0 amu %C = ( 8(12.0 amu) / 152.0 amu ) x 100 = 63.2% Solution to Example 2 C) C7H14O2 FW: 7(12.0 amu) + 14(1.0 amu) + 2(16.0 amu) = 130.0 amu %C = ( 7(12.0 amu) / 130.0 amu ) x 100 = 64.6% Example Problem 3 Caffeine is readily available in common foods and drinks. By mass, caffeine contains 49.48% C, 5.15% H, 16.49% O, and 28.87% N. Its molar mass is 194.2g/mol. A) Determine the empirical formula for caffeine. B) Determine the molecular formula for caffeine. Solution to Example 3 A) Assume the sample is 100g 49.48g C x ( 1 mol C/ 12.011 g C) = 4.1195 mol C 5.15g H x (1 mol H/ 1.00794g H) = 5.1096 mol H 16.49g O x (1 mol O/ 15.9994g O) = 1.03066 mol O 28.87g N x (1 mol N/14.00674g N) = 2.06115 mol N DIVIDE ALL BY THE LOWEST MOLE AMOUNT (1.03066 mole) Gives us: ~4 mol C, 5 mol H, 1 mol O, and 2 mol N Therefore, our empirical formula is C4H5ON2 Solution fo Example 3 B) molecular formula 4(12.011g) + 5(1.00794g) + 15.9994g + 2(14.00674g)= 97.09658 g/ mol empirical 194.2 g/mol / 97.09658 g/mol = 2 Therefore, the empirical equation needs to be double to get the proper molecular formula of caffeine, which is C8H10O2N4. ZE END!