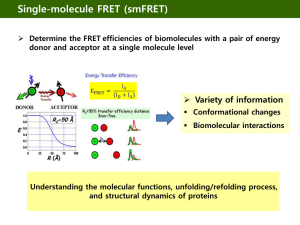
Förster Resonance Energy Transfer (FRET)
advertisement
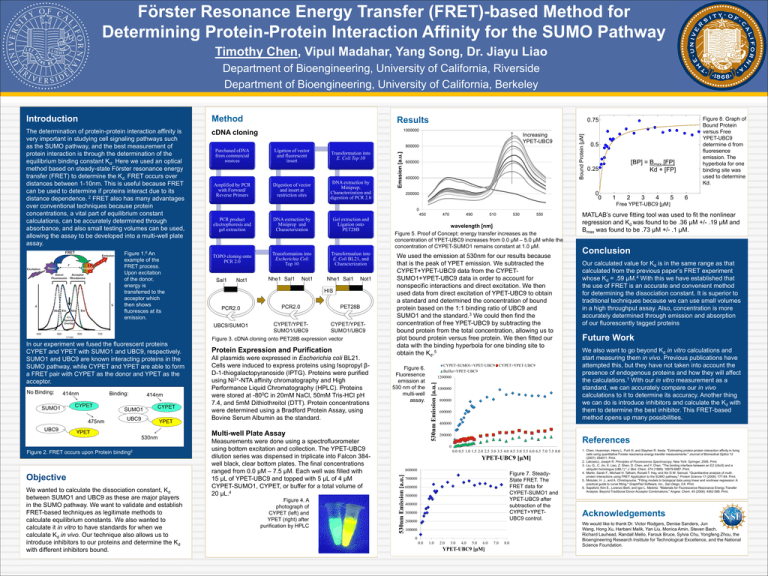
Förster Resonance Energy Transfer (FRET)-based Method for Determining Protein-Protein Interaction Affinity for the SUMO Pathway Timothy Chen, Vipul Madahar, Yang Song, Dr. Jiayu Liao Department of Bioengineering, University of California, Riverside Department of Bioengineering, University of California, Berkeley Method The determination of protein-protein interaction affinity is very important in studying cell signaling pathways such as the SUMO pathway, and the best measurement of protein interaction is through the determination of the equilibrium binding constant Kd. Here we used an optical method based on steady-state Förster resonance energy transfer (FRET) to determine the Kd. FRET occurs over distances between 1-10nm. This is useful because FRET can be used to determine if proteins interact due to its distance dependence. 2 FRET also has many advantages over conventional techniques because protein concentrations, a vital part of equilibrium constant calculations, can be accurately determined through absorbance, and also small testing volumes can be used, allowing the assay to be developed into a multi-well plate assay. cDNA cloning No Binding: 414nm SUMO1 Binding: CYPET 475nm UBC9 414nm SUMO1 CYPET UBC9 YPET YPET 530nm Figure 2. FRET occurs upon Protein binding2 Objective We wanted to calculate the dissociation constant, Kd between SUMO1 and UBC9 as these are major players in the SUMO pathway. We want to validate and establish FRET-based techniques as legitimate methods to calculate equilibrium constants. We also wanted to calculate it in vitro to have standards for when we calculate Kd in vivo. Our technique also allows us to introduce inhibitors to our proteins and determine the Kd with different inhibitors bound. Digestion of vector and insert at restriction sites Transformation into E. Coli Top 10 DNA extraction by Miniprep, Characterization and digestion of PCR 2.0 Emssion [a.u.] Amplified by PCR with Forward/ Reverse Primers Ligation of vector and fluorescent insert 600000 400000 0.5 [BP] = Bmax [FP] Kd + [FP] 0.25 0 0 200000 1 2 3 4 5 6 Free YPET-UBC9 [μM] 0 450 PCR product electrophoresis and gel extraction DNA extraction by Miniprep and Characterization Gel extraction and Ligation onto PET28B TOPO cloning onto PCR 2.0 Transformation into Escherichia Coli Top 10 Transformation into E. Coli BL21, and Characterization Sal1 Not1 Nhe1 Sal1 Not1 Nhe1 Sal1 Not1 HIS PCR2.0 PCR2.0 UBC9/SUMO1 CYPET/YPETSUMO1/UBC9 PET28B CYPET/YPETSUMO1/UBC9 Figure 3. cDNA cloning onto PET28B expression vector Protein Expression and Purification All plasmids were expressed in Escherichia coli BL21. Cells were induced to express proteins using Isopropyl βD-1-thiogalactopyranoside (IPTG). Proteins were purified using Ni2+-NTA affinity chromatography and High Performance Liquid Chromatography (HPLC). Proteins were stored at -800C in 20mM NaCl, 50mM Tris-HCl pH 7.4, and 5mM Dithiothreitol (DTT). Protein concentrations were determined using a Bradford Protein Assay, using Bovine Serum Albumin as the standard. Figure 4. A photograph of CYPET (left) and YPET (right) after purification by HPLC 490 510 530 550 wavelength [nm] Figure 5. Proof of Concept: energy transfer increases as the concentration of YPET-UBC9 increases from 0.0 μM – 5.0 μM while the concentration of CYPET-SUMO1 remains constant at 1.0 μM. We used the emission at 530nm for our results because that is the peak of YPET emission. We subtracted the CYPET+YPET-UBC9 data from the CYPETSUMO1+YPET-UBC9 data in order to account for nonspecific interactions and direct excitation. We then used data from direct excitation of YPET-UBC9 to obtain a standard and determined the concentration of bound protein based on the 1:1 binding ratio of UBC9 and SUMO1 and the standard.3 We could then find the concentration of free YPET-UBC9 by subtracting the bound protein from the total concentration, allowing us to plot bound protein versus free protein. We then fitted our data with the binding hyperbola for one binding site to obtain the Kd.5 Figure 6. Fluoresence emission at 530 nm of the multi-well assay. Multi-well Plate Assay Measurements were done using a spectrofluorometer using bottom excitation and collection. The YPET-UBC9 dilution series was dispensed in triplicate into Falcon 384well black, clear bottom plates. The final concentrations ranged from 0.0 μM – 7.5 μM. Each well was filled with 15 μL of YPET-UBC9 and topped with 5 μL of 4 μM CYPET-SUMO1, CYPET, or buffer for a total volume of 20 μL.4 470 CYPET-SUMO1+YPET-UBC9 CYPET+YPET-UBC9 Buffer+YPET-UBC9 1200000 530nm Emission [a.u.] In our experiment we fused the fluorescent proteins CYPET and YPET with SUMO1 and UBC9, respectively. SUMO1 and UBC9 are known interacting proteins in the SUMO pathway, while CYPET and YPET are able to form a FRET pair with CYPET as the donor and YPET as the acceptor. Increasing YPET-UBC9 800000 Purchased cDNA from commercial sources Figure 8. Graph of Bound Protein versus Free YPET-UBC9 determine d from fluoresence emission. The hyperbola for one binding site was used to determine Kd. 0.75 1000000 1000000 800000 600000 MATLAB’s curve fitting tool was used to fit the nonlinear regression and Kd was found to be .36 μM +/- .19 μM and Bmax was found to be .73 μM +/- .1 μM. Conclusion Our calculated value for Kd is in the same range as that calculated from the previous paper’s FRET experiment whose Kd = .59 μM.4 With this we have established that the use of FRET is an accurate and convenient method for determining the dissociation constant. It is superior to traditional techniques because we can use small volumes in a high throughput assay. Also, concentration is more accurately determined through emission and absorption of our fluorescently tagged proteins Future Work We also want to go beyond Kd in vitro calculations and start measuring them in vivo. Previous publications have attempted this, but they have not taken into account the presence of endogenous proteins and how they will affect the calculations.1 With our in vitro measurement as a standard, we can accurately compare our in vivo calculations to it to determine its accuracy. Another thing we can do is introduce inhibitors and calculate the Kd with them to determine the best inhibitor. This FRET-based method opens up many possibilities. 400000 200000 References 0 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 YPET-UBC9 [μM] 800000 530nm Emission [a.u.] Figure 1.6 An example of the FRET process. Upon excitation of the donor, energy is transferred to the acceptor which then shows fluoresces at its emission. Results Bound Protein [μM] Introduction Figure 7. SteadyState FRET. The FRET data for CYPET-SUMO1 and YPET-UBC9 after subtraction of the CYPET+YPETUBC9 control. 700000 600000 500000 400000 300000 200000 100000 0 0.0 1.0 2.0 3.0 4.0 5.0 6.0 YPET-UBC9 [μM] 7.0 8.0 1. Chen, Huanmian, Henry L. Puhl III, and Stephen R. Ikeda. "Estimating protein-protein interaction affinity in living cells using quantitative Forster resonance energy transfer measurements." Journal of Biomedical Optics 12 (2007): 054011. Print. 2. Lakowicz, Joseph R. Principles of Fluorescence Spectroscopy. New York: Springer, 2006. Print. 3. Liu, Q., C. Jin, X. Liao, Z. Shen, D. Chen, and Y. Chen. "The binding interface between an E2 (Ubc9) and a ubiquitin homologue (UBL1)." J. Biol. Chem. 274 (1999): 16979-6987. Print. 4. Martin, Sarah F., Michael H. Tatham, Ronald T. Hay, and Ifor D.W. Samuel. "Quantitavtive analysis of multiprotein interactions using FRET: Application to the SUMO pathway." Protein Science 17 (2008): 777-84. Print. 5. Motulski, H. J., and A. Christopoulos. "Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting." GraphPad Software, Inc., San Diego, CA. Print. 6. Sapsford, Kim E., Lorenzo Berti, and Igor L. Medintz. "Materials for Fluorescence Resonance Energy Transfer Analysis: Beyond Traditional Donor-Acceptor Combinations." Angew. Chem. 45 (2006): 4562-588. Print. Acknowledgements We would like to thank Dr. Victor Rodgers, Denise Sanders, Jun Wang, Hong Xu, Harbani Malik, Yan Liu, Monica Amin, Steven Bach, Richard Lauhead, Randall Mello, Farouk Bruce, Sylvia Chu, Yongfeng Zhou, the Bioengineering Research Institute for Technological Excellence, and the National Science Foundation.