File
advertisement

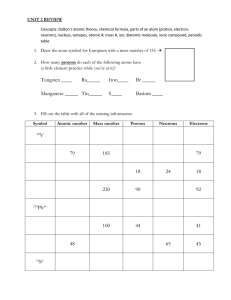
Atoms and Elements Chapter 1 How do we draw atoms? 1. Look at the Atomic number 2. Atomic number = # protons in the atom 3. # protons = # electrons in orbit (we are assuming that the atom is overall neutral, meaning there are the same number of positive charges [protons] as negative charges [electrons]) Example: Carbon Atomic number: 6 6 protons in the nucleus 6 electrons in orbit Orbit/Energy Level Rules We will learn how to draw atoms of the first 20 elements in the periodic table. For the purposes of this course, when drawing Bohr-Rutherford diagrams, use the following as a guide: 1st orbit/energy level : can hold up to 2 electrons 2nd orbit/energy level : can hold up to 8 electrons 3rd orbit/energy level: can hold up to 8 electrons (18 in reality) 4th orbit/energy level: in our class, the max it will ever have is 2 You must fill in the lower levels before you start filling in higher levels Protons go in the nucleus!!!! Example: Nitrogen (N) Atomic Number: # Protons: # Electrons: Example: Calcium (Ca) Atomic Number: # Protons: # Electrons: Drawing Atoms- The Simplified Model The simplified model includes neutrons Flash Back: • Chadwick discovered the Neutron • It is in the nucleus • It holds protons together • It is neutral The number of Neutrons is determined by: Atomic Mass – Atomic # = # neutrons Careful! Atoms can be identified in 2 formulations: Example Magnesium (Mg) Atomic number: 12 Atomic Mass: 24 atomic mass units (u) # Protons: 12 # Electrons: 12 # Neutrons: Atomic mass – Atomic number = 24 – 12 = 12 Example Aluminum (Al) Atomic number: Atomic Mass: # Protons: # Electrons: # Neutrons: Atomic mass – Atomic number = Isotopes • Atoms of the same element always have the same number of protons but may contain different numbers of neutrons. NOTE: Isotopes have the same number of electrons! Isotope: atoms of the same element that have different numbers of neutrons Example: Carbon Most carbon nuclei have 6 neutrons (same # as the # of protons). Some carbon nuclei have 7 or 8 neutrons (not the same # as protons). In order to distinguish between isotopes, scientists refer to them by stating the combined total or protons and neutrons in the nucleus (mass number). Ex: Carbon-12 (has 6 protons, 6 neutrons) Carbon-13 (has 6 protons, 7 neutrons) Carbon-14 (has 6 protons, 8 neutrons) Isotopes Because Isotopes have the same number of protons, but have different amounts of neutrons, their masses are not the same. Reminder: Atomic mass = # protons + # neutrons Isotopes have the same chemical properties but different physical properties. The atomic masses that appear on the periodic table are the average of the masses of isotopes found in nature. The Periodic Table of Elements • Visual representation of the elements in groups according to their properties • Metals are on the left hand side of the staircase, non-metals to the right of the staircase and metalloids are found around the staircase. Metals • On the left side of the staircase (except hydrogen) • Conduct heat and electricity • Ductile (shaped into wires) • Malleable (bendable) • Shiny • Solid at room temperature (except Mercury) • React with acid (produces Hydrogen gas) Non-Metals • On the right side of the staircase (except Hydrogen is a non-metal) • Do not conduct heat or electricity • Many are gases at room temperature (the few that are solids are easily reduced to powder) Metalloids • Found touching the staircase • Have properties of both metals and non-metals • Semiconductors Ex. B, C (sometimes), Si Ge, As, Sb, Te, Po (sometimes) and At Families/Groups of the Periodic Table • They are the columns of the periodic table • ***Hydrogen is not in any group*** • Elements in the same family/group have similar chemical properties Elements in the same family have the same # of valence electrons!!!!!! Valence Electrons: The electrons in the outer most Energy level (orbit) Family/Group # = # of valence electrons Try This! Draw the Bohr-Rutherford atomic models for the first 20 elements in the periodic table. Group IA Alkali Metals (except hydrogen) • 1 valence electron • Soft • React with air and water (Stored in oil) • NEVER found as an element in nature. Always found as compound (attached to another element) in nature. • They get MORE reactive as you go DOWN the column Group IIA Alkaline Earth Metals • 2 valence electrons • Highly malleable • Burn easily • Can be exposed to air • Never found as an element in nature • Get MORE reactive as you go DOWN the column Group VIIA Halogens • 7 valence electrons • Form salts when combined with alkali metal (ex. NaCl) • Form acids when combined with H (ex. HCl) • Disinfectants (Chlorine cleans swimming pools) • Get more reactive as you go UP the column Group VIIIA Noble Gases (Inert Gases) • Stable (react minimally) • CAN be found in nature as an element Periods of the Periodic Table • Period = Horizontal Row of the periodic table • The period # is equal to the # of Energy Levels (Orbits or Shells) of an atom Periodicity of Properties • Several patterns exist in the periodic table according to periods (horizontal rows): boiling point, density, atomic radius, electrical conductivity, etc. • Peaks and troughs characterize these patterns as they occur over and over as you move to the next period • Trends can be explained by the number of particles in the nucleus Atomic Radius • As # protons and electrons increases power of attraction between them increases, which shrinks the atom in size. • Atomic radius INCREASES as you go across a period to the LEFT • Atomic radius INCREASES toward the BOTTOM Chemical Reactivity In metals: • Metals give up electrons in chemical reactions • Reactivity increases as you move to left in period and down in periodic table (this is caused by increase in atomic radius) Larger atomic radius means less attraction between protons and electrons As electrons are more easily removed reactivity increases In non-metals: • Non-metals accept electrons in chemical reactions Reactivity increases as atomic radius gets smaller as positive nucleus attracts electrons more strongly • Reactivity increases toward top of periodic table and toward right in period Ionization Energy Is the energy required to remove a valence electron from its shell. • Energy increases to the right and toward the top of the periodic table (due to atomic radius) • The smaller the atom, the more energy will be required to strip away an electron (attraction to nucleus) Electronegativity Is the force with which an atom holds on to its electrons • Electronegativity increases as atomic radius decreases (smaller atom means greater attraction between protons and electrons) • Electronegativity increases toward right in a period and toward top of periodic table Summary of All Trends Lewis Dot Diagram Is a simplified representation of the atom, in which only Valence Electrons are drawn represented using dots. The group # = # valence electrons = # of dots ***Exception: Helium in group VIIIA only has 2 dots*** Try This! Draw the Lewis Diagrams for the following elements: Na Mg Al C P O Cl Ne Ball and Stick Diagram Atoms are represented with circles and they are attached to one another with sticks. Examples: H2O CH4 Atoms of different elements have different sized/coloured circles