IRB Form: Local Review Board (LRB) Recommendation DePaul
advertisement

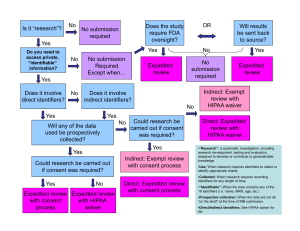
IRB Form: Local Review Board (LRB) Recommendation 1 East Jackson Blvd. Chicago, IL 60604-2201 orp@depaul.edu Ph: (312) 362-7593 http://research.depaul.edu/ DePaul University Office of Research Services Institutional Review Board Version 8/22/2012 See LRB Instructions for additional information. Save As your version before filling this form. I. II. III. LRB Local Review Board: Select One Reviewing Members (at least two required): 1. 2. 3. Project PI Last Name: Project Title: PI First Name: LRB Recommendation – Level of Review A. 1. If recommended for Exemption, which category(ies)? (Note: Prisoner research is not exempt.) 1. Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (i) research on regular and special education instructional strategies, or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods. 2. Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (i) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (ii) any disclosure of the human subjects' responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects' financial standing, employability, or reputation. (Surveys, interviews, and observations where the researcher participates in the observed activity may only be done with adult subjects.) Check for exemption 2: Would disclosure outside the research pose risks related to criminal or civil liability, employability, financial standing, or reputation? Is information obtained recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects? Are children being surveyed or interviewed? Are children being observed during an activity in which the researcher participates? If Yes to both, study is not exempt. No Yes No No Yes Yes If Yes, study is not exempt. No Yes If Yes, study is not exempt. 3. Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior that is not exempt under category 2, if (i) the human subjects are elected or appointed public officials or candidates for public office; or (ii) federal 1 statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter. 4. Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. Check for exemption 4: Do data/documents/records/specimens all currently exist? For privately held data/specimens, will identifiers be recorded? No Yes If No, study is not exempt. No Yes If Yes, study is not exempt. 5. Research and demonstration projects that are conducted by or approved by federal agencies and which study, evaluate, or examine public benefit or service programs. 6. Taste and food quality evaluation and consumer acceptance studies involving food. For complete regulatory citations of exempt categories see: www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html#46.101 2. Exemption application materials The exemption application includes a clear summary of the purpose (aims and Yes No goals), data to be collected, methods, recruitment process, and target population and number. Yes No Participant selection is appropriate for the research question(s). Yes No Any recruitment materials (ads, scripts, e-mails) are included in the application. Yes No Study measures or data collection tools have been included. Yes No An information sheet or appropriate information process is included. Human subjects training has been completed for all research personnel via CITI, Yes No or under the collaborator's own institution, or an alternative process is proposed to meet this requirement. B. If recommended for Expedited Review, the research (1) should present no more than minimal risk; (2) should involve only procedures listed in one or more of the following categories; (3) may not be used for classified research; and (4) may not be used where identification of the subjects and/or their responses would reasonably place them at risk of criminal or civil liability or be damaging to the subjects' financial standing, employability, insurability, reputation, or be stigmatizing, or involve other physical or psychological risks, unless reasonable and appropriate protections will be implemented so that risks related to invasion of privacy and breach of confidentiality, or to the subject in general, are no greater than minimal. Check all that apply: 1. Clinical studies of drugs/medical devices when they do not require an investigational new drug or investigational device exemption application under FDA regulations. 2. Collection of blood by finger stick, heel stick, ear stick, or venapuncture. From healthy adults weighing 110 lbs and not exceeding 550 ml in 8 weeks or more than two times a week. From other adults (considering age, weight, and health) and children, not exceeding 50 ml or 3 ml/kg in 8 weeks or more frequently than 2 times per week. 3. Prospective collection of biological specimens by noninvasive means. 2 4. Collection of data through non-invasive procedures routinely used in clinical practice, excluding x-rays or microwaves. 5. Research involving data, documents, records, or specimens, that have been collected for research or nonresearch purposes, or will be collected for nonresearch purposes. 6. Collection of data using voice, video, or image recordings made for research purposes. 7. Research on individual/group characteristics or behavior or research using survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies. For complete regulatory citations of expedited review categories see: www.hhs.gov/ohrp/policy/expedited98.html C. If recommended for Expedited Review or Full Board Review, answer the following: 1. Risk and Review Level: Minimal risk and qualifies for Expedited Review Minimal risk but requires Full Board Review (does not fit in expedited categories, or involves prisoners) More than minimal risk so requires Full Board Review 2. Is a waiver/alteration of consent or waiver of consent documentation needed? No Yes i. If Yes, what type of wavier/alteration is necessary? (Check all that apply.) Waiver of documentation (signed document) Complete waiver of consent/assent process Alternation of consent/assent (process or elements) Waiver of parent/guardian permission ii. Do the research materials include a Form A-1 and/or A-2, as appropriate? Yes iii. Have the requirements for a waiver/alteration been satisfied? (Refer to Form A-1, A-2.) No Yes No IV. LRB Specific Findings (Evaluate each of the following for expedited and full review protocols.) Yes No 1. The research has a clear purpose, aims, or goals and these have been clearly stated. Yes No 2. The research has scientific merit and uses sound research design appropriate for evaluating the research question(s), so as not to pose unnecessary risks to participants. Yes No 3. The risks to participants are minimized. Yes No 4. Participant selection is appropriate for the research question(s) and is equitable. Yes No 5. Risks to participants are reasonable in relation to anticipated benefits. (Select one or both below.) Yes No The research may directly benefit participants. Yes No The research involves indirect benefit and is likely to contribute to knowledge. Yes No 6. The research includes plans for monitoring data collection to maintain participant safety (unanticipated event/adverse event reporting), as necessary. Yes No 7. The research has adequate plans to protect the privacy of the participants and the confidentiality of the research data. Yes No 8. When applicable, the informed consent documents, parent/guardian permission documents, and assent documents (as applicable to the research) and procedures include all necessary elements. Yes No All consent, parent/guardian permission, and assent forms are readily understandable. Yes No The consent, permission, or assent process assures that participants fully understand the study and is appropriate to the target population. V. Application Completeness, for Expedited and Full Review Protocols Yes No 1. The research application is appropriately completed. Yes No 2. Any recruitment materials (ads, scripts, e-mails) are included in the application. Yes No 3. Study measures or data collection tools have been included. Yes No 4. Human subjects training has been completed for all research personnel and, when 3 VI. VII. Yes No Yes No necessary, documentation is included. 5. As appropriate to the research, all consent, parent/guardian permission, and assent documents are included with the application materials. 6. Where appropriate, collaborative IRB approval and letters of collaboration/cooperation are included. Comments Signatures Your signature indicates that (a) you have personally reviewed the activity recommended and (b) you endorse the proceeding LRB evaluation and recommendations regarding IRB review and waiver/alteration of informed consent. Send the completed Signed form via email to orp@depaul.edu Reviewer 1: __________________________________________ Reviewer 2: __________________________________________ Reviewer 3: __________________________________________ 4