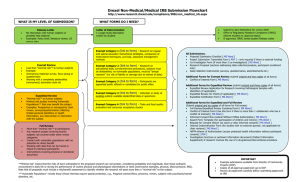
Is it “research”? No submission required Yes No Does it involve
advertisement

Is it “research”? No Yes Do you need to access private, “identifiable” information? Yes No No submission Required Except when… Expedited review Yes Does it involve direct identifiers? OR Does the study require FDA oversight? No submission required No No submission required No Expedited review Indirect: Exempt review with HIPAA waiver Yes Will any of the data used be prospectively collected? No Could research be carried out if consent was required? Yes Yes Could research be carried out if consent was required? Yes Yes No Does it involve indirect identifiers? Yes Will results be sent back to source? No Expedited review Expedited review with consent with HIPAA process waiver Indirect: Exempt review with consent process Direct: Expedited review with consent process Direct: Expedited review with HIPAA waiver •“Research”: a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. •Use: When research requires identifiers to select or identify appropriate charts. •Collected: When research requires recording identifiers for any length of time. •“Identifiable”: When the data contains any of the 18 identifiers (i.e. name, MRN, age, etc.) •Prospective collection: When the data are not all “on the shelf” at the time of IRB submission. •Direct/Indirect identifiers: See HIPAA waiver for list.