IRB Form 4 – Expedited Request
advertisement

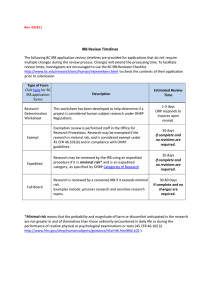
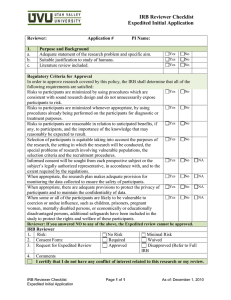
IRB Form 4 Farmingdale State College Institutional Review Board Categories of Research that may be reviewed by the IRB through an Expedited Review Procedure (New Protocols) (Form Approved: February 2007) Date: Does the research involve minimal risk? Yes No Does the research involve the collection of information where identification of the subject’s or their responses could place them at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, insurability, reputation, or be stigmatizing? Yes No If yes, are there protections in place so that risk related to invasion of privacy and breach of confidentiality is no more than minimal? Yes No Does the research involve genetic research? Yes No (If yes, protocol cannot be expedited) Principal Investigator: Protocol Title: In order to qualify for expedited status, the research must involve no more than minimal risk and fall into one of the following categories. For an expanded description of these categories, see IRB Policy 1). Clinical studies of drugs and medical devices when an IND or IDE is not required. Collection of blood samples by finger/heel/ear stick or venipuncture from: ● Healthy, non-pregnant adult (weighing at least 110 lbs.) up to 550 ml in an 8 week period, nor more than 2x per week ● Others, no more than the lesser of 50 ml or 3ml/kg in an 8 week period, nor more than 2x per week. Biological specimens by non-invasive means (hair/nail clippings, deciduous or extracted teeth, excreta and external secretions (e.g. sweat), saliva, placenta, amniotic fluid (at time of rupture), dental plaque, mucosal/skin cells obtained via swabbing, sputum. Data from non-invasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice excluding procedures involving x-rays or microwaves. Research involving materials collected for non-research purposes (data, documents, records, specimens). [Note: Research in this category may qualify as exempt] Data from voice, video, digital or image recordings made for research purposes. Research on individual/group characteristics or behavior, or research involving survey, interviews, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies. [Note: Research in this category may qualify as exempt] _______________________________ Principal Investigator Signature IRB Office Use Only: Expedited Review Request: __ Approved __ Denied Consent Required: __ Yes __ No __________________ Date IRB Reviewer _________________________