Medical Record Review
advertisement

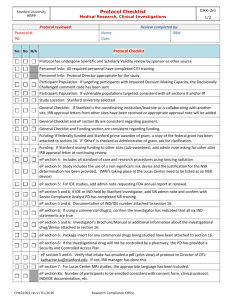
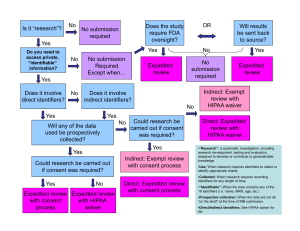
Human Investigation Committee 2013 Is it research? If yes, does it involve human subjects? If yes, can it be exempt? If no, will a Request for Approval of Medical Record Review be the appropriate form to submit? Research: ‘a clinical investigation or a systematic investigation, including research and development, testing and evaluation, designed to develop or contribute to generalizable knowledge’. Quality Improvement activities Activities preparatory to research e.g. reviewing records to determine whether there is a sufficient pool of subjects Case studies Human subject: A living individual (1) about whom an investigator (whether professional or student) conducting research obtains either (a) data through intervention or interaction with the individual; or (b) identifiable private information; or (2) who is or becomes a participant in research involving drugs or devices, either as a recipient of a test article or as a control. Research on decedents Note: A Waiver of HIPAA Authorization may still be needed (contact the HIPAA Privacy Officer) A search of medical records involves looking at identifiable data ◦ Medical records contain highly personal information ◦ Protections for confidentiality are important For the researcher, the exemption means that the project has to be initially reviewed by the IRB but it does not need subsequent annual reviews (but any changes to the project do!) Certain types of research projects can be eligible for a determination of exempt status by the IRB: 1) presenting minimal risk 2) meeting one of the federal categories 45 CFR 46.101(b)(4)Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. All the data or the specimens have to be existing at the time you request exemption You can’t record identifiers A HIPAA waiver will be required if you don’t have a direct healthcare relationship to the potential subjects. If this relates to an investigational drug or device, the study cannot be exempt, per FDA regulations. You are going to search a medical records database for all subjects who had an allergic response to an anesthetic used in the OR from January 2011 through December 2011. You are going to record only: ◦ ◦ ◦ ◦ Their gender Their age Their treatment The type of surgery Retrospective = On the shelf Prospective = medical records have not been created at the time of approval of the request Note: Only retrospective chart review can be exempt Prospective medical records may require a consent from subjects ◦ Consent from Subjects/HIPAA Authorization will be needed unless: The research poses no greater than minimal risk to subjects AND The waiver does not adversely affect subjects’ rights and welfare AND It is impracticable to obtain the subjects consent and authorization for use or disclosure of the PHI without the waiver. The Request for Approval of Medical Record Review form (100 FR 7) should be used for non-exempt research projects involving medical record reviews only, and where there are NO plans to: contact subjects for follow-up, create a data archive for future research. http://www.yale.edu/hrpp/formstemplates/biomedical.html http://www.yale.edu/hrpp/resources/docs/1 00FR7MRR_application8-24-12.doc Check our website: http://www.yale.edu/hrpp/index.html Call the HRPP main number: 785-4688 Or email us at hrpp@yale.edu Thank you!