Chapter 8
advertisement

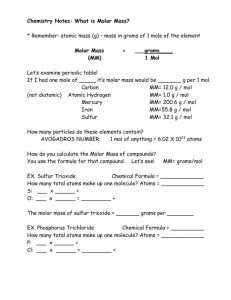
Chapter 8 Mole Conversions Part 2 3.2: Molecular Mass/Weight Formula Mass/Weight •The formula of an Acetylsalicylic Acid (Aspirin) molecule is C9H8O4 •Acetylsalicylic Acid (Aspirin) has molar mass of 180.159 g/mol •How many hydrogen atoms are contained in a 0.372 g tablet? 0.372g C9H8O4 0.372g C9H8O4 mol 180.159 g 0.372g C9H8O4 mol 180.159 g 6.02 x 1023 molecules mol 0.372g C9H8O4 mol 180.159 g 6.02 x 1023 molecules 8 H atoms mol molecule 0.372g C9H8O4 mol 180.159 g 6.02 x 1023 molecules 8 H atoms mol molecule = 9.94 x 1021 H atoms What is the mass in grams of one atom of carbon? What is the mass in grams of one atom of carbon? 12.0 g/mol What is the mass in grams of one atom of carbon? 12.0 g mol What is the mass in grams of one atom of carbon? 12.0 g mol mol 6.02 x 1023 atoms = 1.99 x 10-23 g/atom What is the mass in grams of one molecule of water? What is the mass in grams of one molecule of water? 18.0 g mol What is the mass in grams of one molecule of water? 18.0 g mol -23 g/molecule = 2.99 x 10 mol 6.02 x 1023 molecules Information for Upcoming Mole Quiz • How does a mole relate to particles and mass? (example: One mole of H2O is 6.02 x 1023 H2O molecules and has a mass of 18g). • Be able to use this information in factor-label problems. • Know how to determine the number of atoms in a molecule. Be able to use this information in factorlabel problems. • Be able to determine the number of grams/molecule. • Identify the diatomic gases. • Do metric conversions. Homework • Mole Worksheet (#1-20) • Notice that answers are provided for this worksheet in your notebook.