Chemistry Worksheet: Molarity & Titration Calculations
advertisement
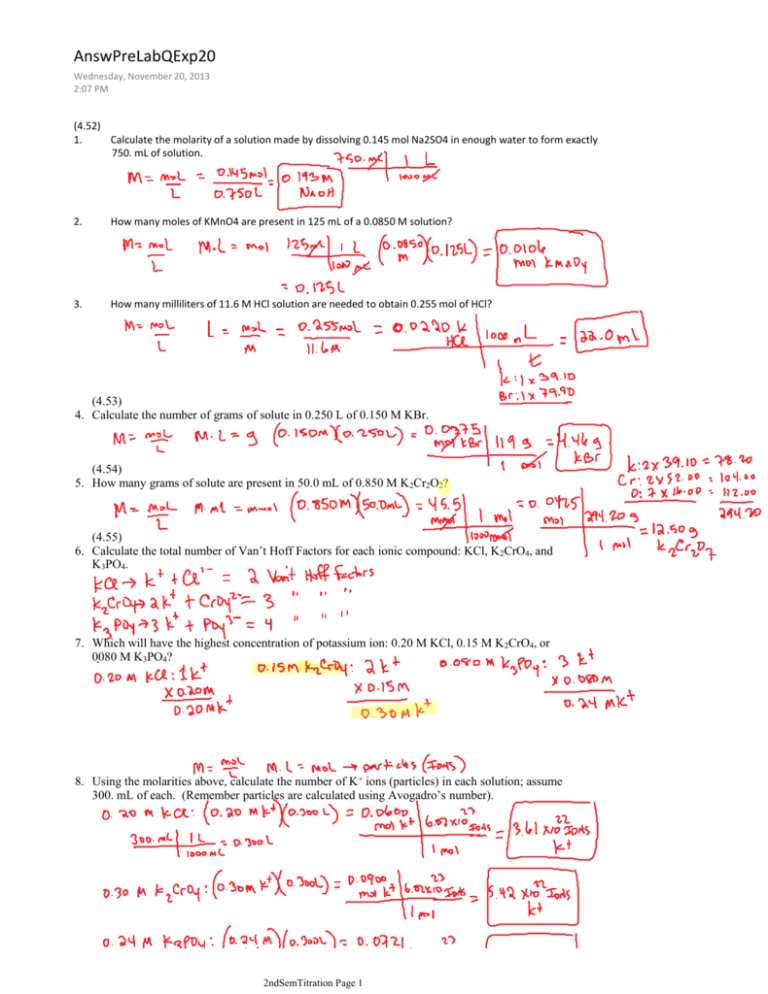
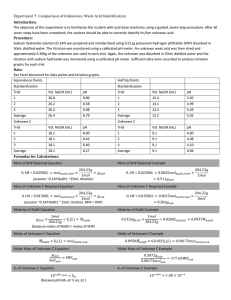
AnswPreLabQExp20 Wednesday, November 20, 2013 2:07 PM (4.52) 1. Calculate the molarity of a solution made by dissolving 0.145 mol Na2SO4 in enough water to form exactly 750. mL of solution. 2. How many moles of KMnO4 are present in 125 mL of a 0.0850 M solution? 3. How many milliliters of 11.6 M HCl solution are needed to obtain 0.255 mol of HCl? (4.53) 4. Calculate the number of grams of solute in 0.250 L of 0.150 M KBr. (4.54) 5. How many grams of solute are present in 50.0 mL of 0.850 M K2Cr2O2? (4.55) 6. Calculate the total number of Van’t Hoff Factors for each ionic compound: KCl, K2CrO4, and K3PO4. 7. Which will have the highest concentration of potassium ion: 0.20 M KCl, 0.15 M K 2CrO4, or 0080 M K3PO4? 8. Using the molarities above, calculate the number of K+ ions (particles) in each solution; assume 300. mL of each. (Remember particles are calculated using Avogadro’s number). 2ndSemTitration Page 1 (4.57) 9. Indicate the concentration of each ion or molecule present in the following solutions: a. 0.14 M NaOH b. 0.25 M CaBr2 c. 0.25 M CH3OH (4.62) 1. How would your prepare 100.0 mL of 0.200 M AgNO3 solution starting with pure solute? 2. An experiment calls for you to use 250. mL of 1.0 M HNO3 solution. All you have available is a bottle of 6.0 M HNO3. How would you prepare the desired solution? (Remember: “Do what you oughta add acid to wataa.” Add acid to water.) 3. Define standardization and state how you would go about doing it. A process to determine the concentration of a solution. As standard of known concentration is titrated according to a known reaction. 4. Define the term titration. Titration is an analytical technique in which one can calculate the concentration of a solute in a solution. 5. Define the term molarity. 6. Why do you weigh by difference? More accurate than weighing directly. 1. What are equivalence points and end points, and how do they differ? Equivalence point is the stoichiometrically equivalent point. Moles of acid = moles of base. 2ndSemTitration Page 2 1. What are equivalence points and end points, and how do they differ? Equivalence point is the stoichiometrically equivalent point. Moles of acid = moles of base. The end point is the point where the indicator changes color. These two point should coincide, but may differ slightly. 2. What is parallax, and why should you avoid it? Parallax should be avoided. It is the apparent difference in apparent direction of an object as seen from two different point not on a straight line with the object. 3. Why is it necessary to rid the distilled water of CO2? (Note: We will not have time for this process.) Forms an acid, could lead to erroneous amount of acid or base (see below). Sodium hydroxide solutions absorb CO2 forming sodium carbonate. 4. If 50.0 mL of NaOH solution is required to react completely with 0.62 g KHP, what is the molarity of the NaOH solution? (Note: At equivalence point moles of acid = moles of base) 1. In the titration of an impure sample of KHP, it was found that 30.6 mL of 0.100 M NaOH was required to react completely with 0.745 g of sample. What is the percentage of KHP in this sample? 2. Calculate the molarity of NaOH using the data below: Mass of bottle + KHP 1.3760 g Mass of bottle 1.0369 g Mass of KHP used ________ g Final buret reading Initial buret reading ml of NAOH used 19.30 mL 4.90 mL ________ g Molarity of NaOH _________ Show your calculations. 2ndSemTitration Page 3 22. Calculate the average molarity. Show your calculations. 0.1161 M 0.1153 M 0.1175 M 23. Calculate the standard deviation using the molarities above. Show your calculations. 23. Calculate the standard deviation using the molarities above. Show your calculations. 2ndSemTitration Page 4