Structure of Solids
advertisement

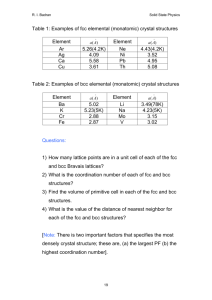
Structure of Solids Objectives By the end of this section you should be able to: • Understand typical ionic crystal structure • Be able to define the primitive unit cell for both graphene and graphite • Differentiate wurtzite and zinc blende structures • Define the perovskite crystal structure & why it’s the most commonly studied oxide structure Metallic Crystal Structures Tend to be densely packed. Several reasons for dense packing: - Metallic bonding is not directional. - Nearest neighbor distances tend to be small in order to lower bond energy. - The “electron cloud” shields cores from each other Metals have the simplest crystal structures. FCC FCC and HCP have very similar lattice energies No clear cut trends Why Aren’t All Systems Close-Packed? • Even with marbles, closed packing is not the only consideration. (Here: confined space) • In crystals: bond distances, types and strengths • Also not all atoms have spherical symmetry Other non close-packed structures • In covalently bonded materials, bond direction is more important than packing What is the atomic # of C? diamond (only 34 % packing) graphite How many bonds would you expect? Not all bonds shown Group: Create Wigner-Seitz cell of this hexagonal lattice How else might you define the unit cell? 1. Pick an origin 2. Draw perp. bisector to all neighboring lattice points 3. Draw smallest area enclosed by bisectors Group: Create Wigner-Seitz cell of the graphene lattice Graphene y α a2 O a) Situation of atoms at the corners of regular hexagons a1 x b) Crystal lattice obtained by identifying all the atoms in (a) 8 Group Exercise • How many atoms are in the primitive unit cell of graphite? Identify a unit cell. Close-packed structures: fcc and hcp hcp ABABAB... fcc ABCABCABC... In groups, build these two differing crystal structures. HCP vs FCC In both the (a) ABA and (b) ABC close-packed arrangements, the coordination number of each atom is 12. Diamond & Zincblende Crystal Structure • Basis set: 2 atoms. Lattice face centered cubic (fcc). • The fcc primitive lattice is generated by r = n1a1+n2a2+n3a3 with lattice vectors: a1 = a(0,1,0)/2, a2 = a(1,0,1)/2, a3 = a(1,1,0)/2 NOTE: The ai’s are NOT mutually orthogonal! Diamond: 2 identical atoms in basis (e.g. 2 C) fcc lattice Zincblende: 2 different atoms in basis and fcc lattice For FCC 2 atom ABCABC stacking, it is called zinc blende For ABAB… stacking it is called wurzite structure (fcc zincblende was ABCABC…) Some compounds can have either structure (i.e., GaN, SiC) Many semiconductors have the Wurtzite Structure Tetrahedral coordination: Each atom has 4 nearest-neighbors (nn). Basis set: 2 atoms. Lattice hexagonal close packed (hcp). A Unit Cell looks like hcp primitive lattice vectors : a1 = c(0,0,1) a2 = (½)a[(1,0,0) + (3)½(0,1,0)] a3 = (½)a[(-1,0,0) + (3)½(0,1,0)] Different planes in FCC Top views Surface unit cells Holes in Close Packed Crystals Two types of holes created by a close-packed arrangement. Octahedral holes lie within two staggered triangular planes of atoms. The coordination number of an atom occupying an octahedral hole is 6. Holes in Close Packed Crystals Tetrahedral holes are formed by a planar triangle of atoms, with a 4th atom covering the indentation in the center. The resulting hole has a coordination number of 4. Surface relaxation • Once the initial slab geometry is set, the system is then subjected to geometry optimization, i.e., the atoms within the supercell are allowed to adjust their positions such that the atomic forces are close to zero • Surface relaxation: a general phenomenon, in which the interplanar distances normal to the free surface change with respect to the bulk value. Surface reconstruction • Relaxation: movement of atoms normal to the surface plane • Reconstruction: movement of atoms along the surface plane The unreconstructed Si (001) surface What kind of crystal structure is this? Surface unit cell Si Surface Reconstruction Reconstructed (001) surface Unreconstructed (001) surface Why does this reconstruction happen? To “passivate” dangling bonds Does the surface have as many nearest neighbors? Ionic materials (Transferred Electron) • In ionic materials, different considerations can be important (electrostatics, different size of ions) • Figure shows the crystal structure of Cs+Cl-. The lattice constant is 4.12 Å and all the bonds shown have the same length. The grey atoms are Cs and the green ones are Cl. • Group: Define crystal structure: meaning what is the primitive Bravais lattice and the associated basis for this crystal (including the locations of these atoms in terms of lattice parameter a)? • What is the angle between the chemical bonds? Cesium Chloride Structure Cs+Cl• Simple cubic lattice with a basis consisting of a cesium ion at the origin 0 and a chlorine ion at the cube center a / 2( x y z ) • CsBr and CsI crystallize in this structure. The lattice constants are in the order of 4 angstroms. NaCl (Rock Salt) Structure • In NaCl the small Na are in interstitial positions between the Cl ions • Group: Define the crystal structure Cs+Cl- For comparison Octahedrals connect interstitial sites Simple Crystal Structures NaCl • NaCl: interpenetrating fcc structures – One atom at (0,0,0) – Second atom displaced by (1/2,0,0) • Majority of ionic crystals prefer NaCl structure despite lower coordination (fewer NN) – Radius of cations much smaller than anions typically – For very small cations, anions can not get too close in the other typical structure (CsCl) – This favors NaCl structure where anion contact does not limit structure as much Predicting Crystal Structures unreliable r cation Coordination # increases with r anion unreliable rNa/rCl = 0.564 moderately reliable quite reliable CsCl ion radius ratio Cesium Chloride structure: rCs rCl 0.170 0.939 0.181 Since 0.732 < 0.939 < 1.0, cubic sites preferred So each Cs+ has 8 neighbor Cl- Define the Crystal Structure of Perovskites A-site (Ca) Oxygen • Superconductors • Ferroelectrics CaTiO3 (BaTiO3) • Colossal Magnetoresistance (LaSrMnO3) B-site (Ti) eg • Multiferroics (BiFeO3) • High εr Insulators (SrTiO3) • Low εr Insulators (LaAlO3) • Conductors (Sr2RuO4) • Thermoelectrics (doped SrTiO3) • Ferromagnets (SrRuO3) t2g Perovskite formula – ABO3 A atoms at the corners B atoms (smaller) at the body-center O atoms at the face centers PEROVSKITES A-site (Ca) Oxygen CaTiO3 B-site (Ti) • Lattice: Simple Cubic (idealized cubic structure) • 1 CaTiO3 per unit cell • Cell Motif: Ti at (0, 0, 0); Ca at (1/2, 1/2, 1/2); 3 O at (1/2, 0, 0), (0, 1/2, 0), (0, 0, 1/2) could label differently • Ca 12-coordinate by O, Ti 6-coordinate by O, O distorted octahedral Is this cube a primitive lattice? Octahedral Tilting How could you measure this? Test 1: Oct 6 (Chapters 1-6 & 8) One page of notes is strongly encouraged. • Test questions will not be as hard as the Ashcroft homework problems, more similar to others • I will not make you solve super hard integrals • Look back at learning objectives from each class I like to test a range of skills, so: • Expect to have to calculate some numbers • Expect to derive some things • Expect some of it to be conceptual • Expect to need to be able to define a crystal structure and/or lattice and its reciprocal