Aldehydes and Ketones
advertisement

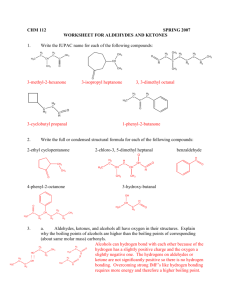
ALDEHYDES AND KETONES By: Dr. Siham Lahsasni ALDEHYDES: STRUCTURE AND NOMENCLATURE General formula: RCHO or RCH=O The aldehyde group is always at the end of a chain IUPAC system: Select the longest continuous carbon chain that contains the C=O group and replace the ending by the suffix al. The CHO group is assigned the number 1 position and takes precedence over other functional groups that may the present such as –OH, C=C for example. O H C H H3C Common Formaldehyde IUPAC H3C Methanal O Cl CH H Acetaldehyde Ethanal HO H3C CH2 C O H Propionaldehyde O C H 2-Chloropropanal O O H3CH2CH2C Butyraldehyde Propanal H Butanal O H3CHC=HC 3-Hydroxypropanal C C H 2-Butenal Aromatic aldehydes are usually designated as derivatives of the simplest aromatic aldehyde, Benzaldehyde. O O H H OH H H O 2N Benzaldehyde O O p-Nitrobenzaldehyde o-Hydroxybenzaldehyde Salicylaldehyde H3CO p-Methoxtbenzaldehyde Anisaldehyde H KETONES: STRUCTURE AND NOMENCLATURE General formula: RCOR’ (R and R’=alkyl or aryl) Common name: listing the alkyl substitutents attached to the carbonyl group, followed by the word ketone. IUPAC system: relpace the ending –e by the suffix –one. The chain is numbred in such a way as give the lowest number to the C=O group. O H3C C O CH3 Common Dimethyl ketone IUPAC H3C C O C6 H 5 Methyl phenyl ketone Acetone Acetophenone Propanone Phenyl ethanone H3C C O CH=CH 2 H5C6 Methyl vinyl ketone C C6H5 Diphenyl ketone Benzophenone 3-Buten-2-one Diphenylmethanone O O C2 H 5 O OH CHO C C2 H 5 Cyclopentylpropanone 3-Ethyl-2-hydroxycyclohexanone 5-Oxohexanal PHYSICAL PROPERTIES OF KETONES AND ALDEHYDE O C + O C O C - C O Because the polarity of the carbonyl group, aldehydes and ketones are polar compounds. Dipole-dipole attractions, although important, are not as strong as intractions due to hydrogen bonding. As a result, the boiling points of aldehydes and ketones are higher than those of nonpolar alkanes, but lower than those of alcohols. C O H O H O C The lower aldehydes and ketones are soluble. PREPARATION OF ALDEHYDES AND KETONES 1- Oxidation of alcohols RCH 2 OH CrO 3/ pyridine O R Cu / heat H O CrO 3/ pyridine R2CH R OH C R Cu / heat 2- Reduction of acid chloride H2 / Pd(BaSO4) O R-H 2C-C R-CH2-CHO Cl O O LiAlH[O(CH3)3] 3 Cl H 3- Ozonolysis of alkenes A A A 1)O 3 A O 2)Zn / H 2O A A + A O A 4- Hydration of alkynes H C C + HO H H2SO4, HgSO4 H C C OH an enol unstable C C H O carbonyl more stable -78 C H 3C C CH CH3-CH=CH (Sia)2BH + ether O H 2O2/ OH H 2O CH3-CH 2-CH CH 3 CH 3 Sia= CH 3-C C H H : disiamyl (Sia) 2BH 5- Friedel Grafts acylation O O + CH3 AlCl 3 R Cl 6-Oxo reaction - Hydroformylation reaction CH3-CH=CH2 + H2 + CH3-CH2-CH2-CHO CO CH3-CH-CH3 + CHO 7- Gattermann-Koch reaction 75 % 25 % CHO + - C O+ + HCl + AlCl3 CO H-C H+ O+ H-C+ CHO + H-C+ O O 8- Oxidation of an Alkyl Side of aromatic ring H3 COCO CH 3 OCOCH3 HC CHO H2O / H+ CrO3 / 10 C (CH3CO)2O 9- From acid chloride and lithium dialkyl cuperate or R2 cd O R C O Cl + R2CuLi O C Cl + (CH3-CH2)2CuLi -78 C ether -78 C ether O H3 C C R C R2 O C CH2 -CH 3 O Cl + (Ph)2Cd -78 C ether C CH 3 10- From Carboxylic acid and RLi O R O C 1) Ether 2 R'Li + OH R C R' + R'H + 2 LiOH 2) H3O+ O O C OH + 1) Ether 2 CH3Li C CH3 2) H3O+ 11- From nitrile and Grignard reagent or alkyl lithium O NMgX R C N H3O+ Ether + R'MgX R C R R' C N + R'Li H3O+ Ether R C R' R C O H 3C H 3C H C C N + PhLi 1) Ether 2) H3O+ R' O NLi R C C R' CH 3 H C CH 3 REACTIONS OF ALDEHYDES AND KETONES H R R C+ O - H C+ > O - C+ > O - R' H Activity of the carbonyl group Nu- C+ O - Nu C O- E+ Nu C OE 1- reduction of carbonyl group a- Addition of metal hydride C O + M+H- H C H2O H OAl O + H C OH OH H2O LiAlH4 H 2 H 2 / Pd H3C O -M+ H+ OH H3C O H 1) NaBH 4 2) H 2O H3C OH b- Addition of Grignard Reagents: Formation of alcohols R' O R + C R'MgX H 1) Dry ether R HO + C OH 2) H 2O O H3C CH C2H5MgX H 1) Dry ether 2) H 2O H3C CH C2H5 R' O R C R' + R''MgX 1) Dry ether 2) H 2O R C OH R'' CH3 O + CH 3MgX 1) Dry ether 2) H 2O OH c- Clemmenson reduction O H COOH H HCl / Zn(Hg) COOH d- The Wolf-kishner reduction O N-NH 2 NH2NH2 COOH COOH H NaOH H COOH e- Wittig reaction O C + CH2=P(C6H5)3 O P(C 6H 5) 3 C CH 2 O- P(C 6H 5) 3 C CH 2 THF C CH 2 (C6H5)3P=O + O O H + (Ph)3P=CH C OC 2 H5 O OC2 H 5 2- Oxidation reaction aR-CHO or Ar-CHO KMnO4 or RCOOH or K Cr O 2 2 7 ArCOOH b- Tollenis test RCHO + 2 Ag(NH3)+2 + OH- RCOO- + 2 Ag + 4 NH3 + 3 H2O c- Iodoform reaction O H3C C O + 3 I2 + 4 NaOH R O Na R CH3 H3C O I 2 / NaOH - H3C COONa + + CHI3 + + CHI3 3 NaI 3- Addition of Hydrogen Cyanide: Formation of cynohydrins R' O R C + R' R HCN C OH CN Cyanohydrin CN O H NH2 OH + OH H2 / Pt + or LiAlH 4 and H 3O HCN Benzaldehyde cyanohydrin O OH + H3O CN HCN OH + COOH Heat 4- Addition of acetylide ions: R' O R C R' + - 2 C Na R C H3 O + + R C C C R OH O + H3C C - C Na + H3O + OH C C CH3 2 5- Addition of alcohols: R'O O R C 2 R =H: 2 R =Alkyl + 2 R'OH H R R''OH C OH R Aldehyde R Hemiacetal Ketone Hemiketal H + C2H5OH H3C C H 5C 2O CH OC 2H5 CH3 C2H5OH C2H5OH + H H3C C OC 2H5 H5C2O H H3C CH Acetal HO + + 2 Ketal Hemiacetal H3C OR'' R Acetal H H O C HO + C R + 2 O H3C R'O + OC 2H5 CH3 Hemiketal C2H5OH + H H3C C CH3 Ketal OC 2H5 6- Addition of Ammonia and Ammonia Derivatives NH3 C NH Imine NH 2OH Hydroxylamine H2N C N OH Oxime NH2 C Hydrazine C N NH2 Hydrazone O H2N NH C Phenylhydrazine NO 2 O 2N NO 2 NH C NH C Semicarbazide NH N - O H2N NH Phenylhydrazone O 2N H2N N NH2 2,4 Dinitrophenylhydrazone O C N NH C Semicarbazone NH2 7- Aldol condensation The reaction occurs in any aldehyde or ketone containing α hydrogen: 2 O H3C 2 dil. NaOH C O R C OH CH2 C R R CH3 CH3 H3C dil. NaOH CH3 H3C O O OH CH3 O O O OHCH3 O CH2 CH3 - O O- H2O OH 8- Cannizzaro reaction Aldehyde which does not contain α hydrogen undergoes Cannizzaro reaction. CHO CH 2 OH NaOH (30 %) + COO-Na+ + O O O- CH C + C H OH OH- H O O C CH2 OOH CH2 OH C + O- more stable anion +