Lipids
advertisement

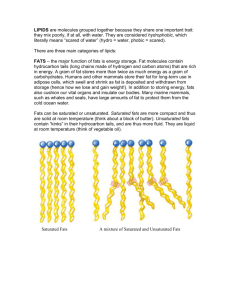
Lipids Chapter 16 What are Lipids? • Lipids = are a family of chemical compounds that are a main component in every living cell. They include the following 3 categories: – Triglycerides – largest class of lipids. They include nearly all of the fats and oils people typically eat. (of the lipids 95% are fats and oils) – Phospholipids - these lipids have a structure that enables them to dissolve in both fat and water. – use as an emulsifiers (found in eggs and peanuts) – Sterols – compound, bile acids and certain hormones perform vital functions. Both plant and animal food contain sterols, but food contains cholesterol (a sterol) Triglycerides • Main function is to fuel the body and keep it warm. • Adipose Tissue = pockets of fat-storing cells – Also adipose tissue under each kidney forms a cushion to help protect it from impact. – Natural body oils help keep supple and hair glossy and healthy. – Fat also carries certain vitamins in the body. Structure of Triglycerides • Triglycerides are composed of carbon, hydrogen, and oxygen. Fatty Acids • Organic compounds that contain a carboxyl group at one end are classified as organic acids or fatty acids. • These fatty acids are organic compounds that have a carbon chain with attached hydrogen atoms and carboxyl group at one end. • Carboxyl group = carbon bonded to oxygen by a double covalent bond, and to a hydroxyl group with a single bond. – COOH – Not all fatty acids are the same, the length of the carbon chain makes the different. – To form triglycerides (tri meaning 3), three fatty acids react with the alcohol glycerol – The human body can make all but two fatty acids, linoleic and linolenic. » These are needed for normal growth and development are obtained from foods as vegetables, grains, nuts, seeds, and soybeans. Saturated vs. Unsaturated Fat • Saturated fat – most of the fatty acids are saturated, in other words, the fatty acids contain all their hydrogen atoms their molecular structure can hold, making 4 single bonds. • Single bond – covalent bond in which each atom donates only one electron to form the bond (sharing one pair of electrons between them) • Unsaturated fat – most of the fatty acids are also unsaturated, meaning they’re missing hydrogen • When hydrogen atoms are missing, a single bond cannot form, to make up the difference a double bond forms • Double bond – covalent bond in which each atom donates two electrons to form the bond Unsaturated fat continued • Unsaturated fats are classified by the number of hydrogen atoms that “drop out” and produce double bonds – Monounsaturated fat – lacks two hydrogen's, which creates one double bond between carbons ex oleic acid or avocados, canola and peanut oil – Polyunsaturated fat – has two or more double bonds between carbons ex linoleic acid or nuts, sesame seed and sunflower oils Properties of Triglycerides Energy Value – The structure of triglycerides makes them bundles of energy – Energy is released by oxidation – Triglycerides supply over twice the energy of glucose – 9 kcalories per gram compared to 4 Solubility – Slightly soluble in water Phase Differences – Fats which usually come from animals are saturated – their single bonds allow them a full set of hydrogen's, which makes them solid at room temperature – Most plant oils are unsaturated, the molecule forms a double bond making the oils less compact, so they are liquid at room temperature Properties of Triglycerides Continued Melting Range – Molecular structure has related effect on how fats change • Most substances have a melting point and a freezing point • Fatty acids with more carbon have higher melting point ex olive oil Solidification Point = the temperature at which melted fat regains its original firmness • Ex you put carrots you cooked in butter the night before in your refrigerator, the next day they are coated with firm butter flecks Functions of Fats in Food Tenderizing • One of the most important uses of fats in foods is tenderizing baked goods (fat coast the flour, creating a flaky, delicate, lighter texture) Aeration • Add air or gas to batters and dough's – this decreases viscosity, making the batter flow more easily Emulsions • Lipids play two roles in creating emulsion, they might be part or they might influence one to form. • Ex. adding mayonnaise creates an emulsion between oil and vinegar • Triglycerides can be broken down into monoglycerides, these have one fatty acid attached and are stable enough to emulsify other fats with liquids Flavor • Fats carry flavor, they dissolve aromatic molecules in food and distribute their essence through out the recipe – Ex. bacon is a salty fat, olive oil is mildly flavored Oxidation • The oxidation of fats occur as the surface of foods react with oxygen. • When fatty food oxidize, they lose electron which is a main reason that high-fat foods spoil • Oxidation involves the loss of a hydrogen atom from a singlebonded carbon, this is why unsaturated fats are more prone to oxidation because they have more double bonds • Heat speeds the rate of oxidation, cooling slows but doesn't stop the process • Water prevents slow oxidation as well by preventing contact between substances and elemental oxygen • Rancid = the term that describes the unpleasant flavors that develop as fats oxidize » Rancidity produces a distinctive flavor, but different for each type of food Commercial Uses of Fats • Commercially important fats fall into two main groups: animal fats and plant oils • Animal Fats • Butter is a natural emulsion, 80% fat and 18% water, remaining 2% is the protein that binds the other two ingredients • Lard or rendered hog fat is popular in baking • Beef fat is usually combined with vegetable fats for uses in food • Plant Fats • Plant lipids are derived from certain oil-rich seeds, in liquid form they are often called vegetable oil ex corn, olive, canola and oil blends • Hydrogenated Oils • Ex. vegetable shortening • Hydrogenation = a chemical process in which hydrogen is added to unsaturated fat molecules, breaking some double bonds and replacing them with single bonds – Partial hydrogenation changes liquid oil to spreadable, semisolid fat – Hydrogenation adds stability by eliminating some of the double bonds in fatty acids – Hydrogenated oil resists rancidity better than liquid oil because it doesn't develop a stale flavor and odor as quickly Cooking with Fat Effects on Fats – Repeated exposure to intense heat causes a decomposition similar to oxidation – Also foods release some of their own fat, water and other substances into the frying oil, which deteriorates the fat, a process called cracking – Cracking can discolor oil and produce off flavors and odors – Smoke point = the temperature at which a fat produces smoke » Reaching a smoked point is part of a cycle of fat breakdown: smoking makes the fat less stable » Vegetable oils generally have a higher smoke point than animal fats, making them more useful for frying Frying Safety – Safe frying starts with the correct equipment (a deep-fat fryer or a frying pan with heavy-duty metal sides) – Food needs to be dry – WHY – Using the correct amount of oil, if oil spills out of the pan you need to shut off heat IMEDIATELY – How do you put out a grease fire? Triglycerides in the Diet • Fats in food are broken down into fatty acids and glycerol, then absorbed by villi in the small intestine • Some of these fatty acids are stored in the liver, remaining fats end up as adipose tissue (tissue designed to store fat) • A typical American diet gets 45% of its calories from fat, well over the 30% recommended (Recommended daily value limit is 65g for fat and 20g saturated fat) Low-Fat Options • According to a recent survey, as many as 56% of American adults are trying to limit their fat intake • You have to be careful, because often low-fat and fatfree foods are often high in calories Triglycerides in the Diet Continued • Fat Replacers • Fat replacers are loosely grouped as either fat, protein, or carbohydrate based – Fat-based replacers are manufacture from, very short and long carbon-chain acids, which supply fewer kcalories – Protein-based replacers use milk or protein particles to stabilize and texturize dairy products and some baked goods, sauces, and soups – Carbohydrate fat replacers include cellulose, gums, and modified food starch • Trans Fat (also known as trans fatty acids) • Is a manmade type of fat formed when a liquid vegetable oil is made into solid fat, like margarine • Trans fat is made when hydrogen is added to vegetable oil in a process called hydrogenation • Hydrogenation is popular because it increases the stability and shelf life of foods • Partial hydrogenation – is when some of the double bonds and the hydrogen atoms end up on different sides of the chain • Trans fat behaves in the human body much like a saturated fat » It raises the LDL or bad cholesterol » Found in foods like vegetable shortening, some margarines, cookies, snack foods, fried foods, baked goods, and other processed foods made with partially hydrogenated veg. oils Cholesterol • Cholesterol = is not a triglyceride, but a sterol, a fatty alcohol made from glucose or saturated fatty acids • Formula is C27H45OH • Cholesterol is vitamin in producing vitamin D and some hormones • The liver makes all the cholesterol you need • Cholesterol in the blood is though to contribute to the manufacture of plaque = a mound of lipid material mixed with calcium and smooth muscle cells – Atherosclerosis (hardening of the arteries) = buildup of plaque along the inner walls of the arteries LDL and HDL Cholesterol • Lipoproteins = large, complex molecules of lipids and protein that carry lipids in the blood • LDL – low-density lipoproteins, they carry about 75% of cholesterol in the blood » Bad cholesterol (want a low number) • HDL –high-density lipoproteins – they return cholesterol to the liver for breakdown and disposal » Good cholesterol ( want a high number) Omega 3 Fatty Acids • Scientists believe that omega-3 fatty acids, found in some fish, promote heart heath in two ways: • First they make it more difficult for plaque to form or clump • They also make plaque less sticky and less likely to collect in the arteries • Sardines, salmon, tuna, herring, and other ocean fish are highest in omega-3 fatty acids or cod liver oil capsules and marine oils Questions 1. 2. 3. 4. Describe the tree categories of lipids. What functions do triglycerides serve in the body? Describe the structure of triglycerides. Why does the body need to obtain linoleic and linolenic acids from plant sources? 5. How are unsaturated fats classified? Give examples. 6. How do fats compare to carbohydrates as an energy source? Questions Continued 7. How does carbon bonding affect the phase of a fat? 8. Why do fats have a melting range rather than a melting point? 9. From a chemical standpoint, why might stale potato chips become rancid? 10. How does hydrogenation affect oils? 11. If you fried chicken in a restaurant, what signs might tell you the frying oil needed changing? 12. Compare low-and high-density lipoproteins. 13. How do marine oils differ from animal fats?