Project Overview
advertisement

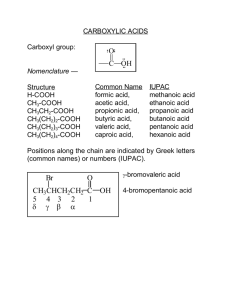
Chapter 17 Carboxylic Acids and Their Derivatives Nucleophilic Addition–Elimination at the Acyl Carbon Created by Professor William Tam & Dr. Phillis Chang Ch. 17 - 1 About The Authors These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in research and teaching, and according to Essential Science Indicators, he is currently ranked as the Top 1% most cited Chemists worldwide. He has published four books and over 80 scientific papers in top international journals such as J. Am. Chem. Soc., Angew. Chem., Org. Lett., and J. Org. Chem. Dr. Phillis Chang received her B.Sc. at New York University (USA) in 1994, her M.Sc. and Ph.D. in 1997 and 2001 at the University of Guelph (Canada). She lives in Guelph with her husband, William, and their son, Matthew. Ch. 17 - 2 1. Introduction Carboxylic Acid Derivatives O O O OH R carboxylic acid R' O R acid anhydride Cl R acid chloride O O R OR' ester O R NR'2 amide Ch. 17 - 3 2. Nomenclature and Physical Properties Nomenclature of Carboxylic Acids and Derivatives ● Rules Carboxylic acid as parent (suffix): ending with “–oic acid” Carboxylate as parent (suffix): ending with “–oate” Ch. 17 - 4 Most anhydrides are named by dropping the word acid from the name of the carboxylic acid and then adding the word “anhydride” Acid chloride as parent (suffix): ending with “–oyl chloride” Ester as parent (suffix): ending with “–oate” Amide as parent (suffix): ending with “amide” Nitrile as parent (suffix): ending with “nitrile” Ch. 17 - 5 Examples O O OH Ethanoic acid (acetic acid) O O O Ethanoic anhydride (acetic anhydride) OCH3 Methyl propanoate O NH'2 Ethanamide Ch. 17 - 6 Examples O O O Cl Na Sodium benzoate Benzoyl chloride H3C C N Ethanenitrile Ch. 17 - 7 2C. Acidity of Carboxylic Acids O R O H pKa ~ 4-5 Compare ● pKa of H2O ~ 16 ● pKa of H2CO3 ~ 7 ● pKa of HF ~ 3 Ch. 17 - 8 When comparing acidity of organic compounds, we compare the stability of their conjugate bases. The more stable the conjugate base, the stronger the acid pKa CH3COOH CH3CH2OH 4.75 16 Ch. 17 - 9 O CH3 O O H + H2O A1 CH3CH2 A2 CH3 O + H3O+ B1 O H + H2O CH3CH2 O + H3O+ B2 Ch. 17 - 10 The conjugate base B1 is more stable (the anion is more delocalized) than B2 due to resonance stabilization O CH3 O O CH3 O O CH3 O ● Thus, A1 is a stronger acid than A2 Ch. 17 - 11 Acidity of Carboxylic Acids, Phenols and Alcohols O O O pKa = 4.20 H O H H pKa = ~ 10 pKa = ~ 17 Ch. 17 - 12 Acidity of Carboxylic Acids, Phenols and Alcohols O O O H O + H2O + H3O+ O O Ch. 17 - 13 Acidity of Carboxylic Acids, Phenols and Alcohols O O H + H3O+ + H2O O O O Ch. 17 - 14 Acidity of Carboxylic Acids, Phenols and Alcohols O H O + H2O + H3O+ (NO resonance stabilization) Ch. 17 - 15 Question If you are given three unknown samples: one is benzoic acid; one is phenol; and one is cyclohexyl alcohol; how would you distinguish them by simple chemical tests? ● Recall: acidity of O O O H O H H > > Ch. 17 - 16 O O R O H + Na OH R + H2O O Na (soluable in water) O H O Na + NaOH (soluble in water) O (immiscible with H2O) H + NaOH No Reaction Ch. 17 - 17 O O O H + NaHCO3 O Na + CO2(g) + H2O (gas evolved) O O H H + NaHCO3 No Reaction + NaHCO3 No Reaction Ch. 17 - 18 O O Cl OH Cl Cl > pKa H 0.70 > H OH H H 1.48 OH H 4.76 2.86 Stability of conjugate bases O O Cl Cl > Cl O > Cl OH H Cl Cl O Cl H Cl O > Cl O > H H O O O O > H H H Ch. 17 - 19 O O > Cl > OH Cl 2-Chlorobutanoic acid (pKa = 2.85) OH 3-Chlorobutanoic acid (pKa = 4.05) O Cl OH 4-Chlorobutanoic acid (pKa = 4.50) Ch. 17 - 20 2D. Dicarboxylic Acids pKa (at 25oC) Common Name mp (oC) pK1 pK2 Oxalic acid 189 dec 1.2 4.2 HO2CCH 2CO2H Malonic acid 136 2.9 5.7 HO2C(CH 2)4CO2H Adipic acid 153 4.4 5.6 Phthalic acid 206-208 dec 2.9 5.4 Structure HO2C CO2H CO2H CO2H Ch. 17 - 21 2J. Spectroscopic Properties of Acyl Compounds IR Spectra ● ● The C=O stretching band occurs at different frequencies for acids, esters, and amides, and its precise location is often helpful in structure determination Conjugation and electron-donating groups bonded to the carbonyl shift the location of the C=O absorption to lower frequencies Ch. 17 - 22 IR Spectra ● ● ● Electron-withdrawing groups bonded to the carbonyl shift the C=O absorption to higher frequencies The hydroxyl groups of carboxylic acids also give rise to a broad peak in the 2500-3100-cm-1 region arising from O– H stretching vibrations The N–H stretching vibrations of amides absorb between 3140 and 3500 cm-1 Ch. 17 - 23 Ch. 17 - 24 Ch. 17 - 25 1H NMR Spectra ● The acidic protons of carboxylic acids are highly deshielded and absorb far downfield in the d 10-12 region ● The protons of the a carbon of carboxylic acids absorb in the d 2.0-2.5 region Ch. 17 - 26 Ch. 17 - 27 13C NMR Spectra ● The carbonyl carbon of carboxylic acids and their derivatives occurs downfield in the d 160-180 region (see the following examples), but not as far downfield as for aldehydes and ketones (d 180220) ● The nitrile carbon is not shifted so far downfield and absorbs in the d 115-120 region Ch. 17 - 28 13C NMR chemical shifts for the carbonyl or nitrile carbon atom H3C O O O C C C H3C OH H3C OEt d 170.3 d 170.7 d 177.2 Cl O H3C C NH2 d 172.6 H3C C N d 117.4 Ch. 17 - 29 3. Preparation of Carboxylic Acids By oxidation cleavage of alkenes ● Ph Using KMnO4 1. KMnO4, OH, heat 2. H3O+ OH Ph ● O + OH Using ozonolysis 1. O3 O HO O OH O 2. H2O2 Ch. 17 - 30 By oxidation of aldehydes & 1o alcohols ● e.g. H OH 1. Ag2O 2. H3O+ O O OH OH 1. KMnO4, OH, heat O + 2. H3O O O H or OH H2CrO4 OH Ch. 17 - 31 By oxidation of alkyl benzene O R 1. KMnO4, OH, heat OH 2. H3O+ (R = 1o or 2o alkyl groups) Ch. 17 - 32 By oxidation of benzene ring ● e.g. 1. O3, CH3COOH 2. H2O2 O OH Ch. 17 - 33 By hydrolysis of cyanohydrins and other nitriles ● e.g. O O Ph HCN CH3 Br NC Ph HCN H+ OH CH3 H2O + CN H H2O, heat HO Ph C OH CH3 O C OH Ch. 17 - 34 By carbonation of Grignard reagents ● e.g. Br Mg MgBr Et2O 1. CO2 2. H3O+ O OH Ch. 17 - 35 4. Acyl Substitution: Nucleophilic Addition-Elimination at the Acyl Carbon O O + R Nu Y R Nu Y O Y + R Nu (Y = leaving group, e.g. OR, NR2, Cl) This nucleophilic acyl substitution occurs through a nucleophilic addition-elimination mechanism Ch. 17 - 36 This type of nucleophilic acyl substitution reaction is common for carboxylic acids and their derivatives O O R OH carboxylic acid O R Cl acid chloride OR' ester R O R' acid anhydride O O R O R NR'2 amide Ch. 17 - 37 Unlike carboxylic acids and their derivatives, aldehydes & ketones usually do not undergo this type of nucleophilic acyl substitution, due to the lack of an acyl leaving group O A good leaving group R Y a carboxylic acid derivative O R O H R R' Not a good leaving group Ch. 17 - 38 Relative reactivity of carboxylic acid derivatives towards nucleophilic acyl substitution reactions ● There are 2 steps in a nucleophilic acyl substitution The addition of the nucleophile to the carbonyl group The elimination of the leaving group in the tetrahedral intermediate Ch. 17 - 39 ● Usually the addition step (the first step) is the rate-determining step (r.d.s.). As soon as the tetrahedral intermediate is formed, elimination usually occurs spontaneously to regenerate the carbonyl group ● Thus, both steric and electronic factors that affect the rate of the addition of a nucleophile control the reactivity of the carboxylic acid derivative Ch. 17 - 40 ● Steric factor e.g. reactivity of O O Cl > Cl ● Electronic factor The strongly polarized acid derivatives react more readily than less polar ones Ch. 17 - 41 ● Thus, reactivity of O O O O > R Cl most reactive O > R O R' > R OR' R NR'2 least reactive ● An important consequence of this reactivity It is usually possible to convert a more reactive acid derivative to a less reactive one, but not vice versa Ch. 17 - 42 5. Acyl Chlorides 5A. Synthesis of Acyl Chlorides Conversion of carboxylic acids to acid chlorides O O R OH R Cl ● Common reagents SOCl2 (COCl)2 PCl3 or PCl5 Ch. 17 - 43 ● Mechanism O Cl Cl O O O O R R OH O Cl R O Cl Cl O O Cl O O O R Cl O O Cl O O R Cl + CO2 + CO + Cl Ch. 17 - 44 Nucleophilic acyl substitution reactions of acid chlorides ● Conversion of acid chlorides to carboxylic acids O R Cl + H2O O base R OH Ch. 17 - 45 ● Mechanism O R OH O H2O Cl R O Cl H + R R OH Cl H H O B H B: OH R O OH Ch. 17 - 46 ● Conversion of acid chlorides to other carboxylic derivatives O R'OH pyridine O R (ester) R O R'2NH Cl OR' (amide) R NR'2 O R' O O Na O (acid anhydride) R O R' Ch. 17 - 47 6. Carboxylic Acid Anhydrides 6A. Synthesis of Carboxylic Acid Anhydrides O O + + R OH R' Cl N O O + R O R' Cl N H Ch. 17 - 48 O O O O + Na Cl + R O Na R' O Cl R O R' O 300oC OH O + H2O OH Succinic O acid O Succinic anhydride O O OH OH Phthalic acid O 230oC O Phthalic anhydride O (~100%) + H2O Ch. 17 - 49 6B. Reactions of Carboxylic Acid Anhydrides Conversion of acid anhydrides to carboxylic acids O R O O R' + H2O O + H O + R OH HO R' Ch. 17 - 50 ● Mechanism O R O O O H+ R' R H O OH O R' H2O R H O R H2O OH H R O O O H OH O OH + R'COOH R OH R' O O R' H Ch. 17 - 51 Conversion of acid anhydrides to other carboxylic derivatives O R'OH R O R O OR' + R OH O O R' O R2'NH R O NR'2 + R O NR'2H2 Ch. 17 - 52 7. Esters 7A. Synthesis of Esters: Esterification O + R'OH R OH O + H + H2O R OR' Ch. 17 - 53 Mechanism O R H+ O OH H H R O "activated" O R O H H2O OR' H R O R'OH R OH O H R' OH2 OR' R HO OR' Ch. 17 - 54 e.g. Esters from acyl chlorides O Cl Benzoyl chloride + EtOH + N O OEt Ethyl benzoate (80%) + Cl N H Ch. 17 - 55 Esters from carboxylic acid anhydrides e.g. O O OH + O Acetic anhydride Benzoyl alcohol O O Benzoyl acetate O + OH Ch. 17 - 56 7B. Base-Promoted Hydrolysis of Esters: Saponification Hydrolysis of esters under basic conditions: saponification O R O OH OR' H2O + R R'OH O Ch. 17 - 57 Mechanism O O R OR' OH R HO OR' O R H+ O R OH O H + OR' O R'OH + R O Ch. 17 - 58 Hydrolysis of esters under acidic conditions O R O H+ OR' H2O + R R'OH OH Ch. 17 - 59 Mechanism O R O H+ OR' R H OR' OH H2O R H O R H2O OH H R O OR' H OH O OH + R'OH R OH O R' H Ch. 17 - 60 7C. Lactones Carboxylic acids whose molecules have a hydroxyl group on a g or d carbon undergo an intramolecular esterification to give cyclic esters known as g- or d-lactones Ch. 17 - 61 R d g O + H O O O OH H HO O a d-hydroxyacid A H O A H H O H O R H + H H R O O H O O + O H H H R a d-lactone R Ch. 17 - 62 Lactones are hydrolyzed by aqueous base just as other esters are O O H+/H2O O C6H5 HA, slight excess C6H5 OH 0oC HA, exactly 1 equiv. O C6H5 O OH OH Ch. 17 - 63 8. Amides 8B. Amides from Acyl Chlorides O O R R Cl Cl H N :NHR'R" R" R' O Cl + R'R"NH2 + R O N R' R" R R"R'HN: R" N R' :Cl: H Ch. 17 - 64 8C. Amides from Carboxylic Anhydrides O O H + 2 R O R N R' R" O R H O N R' + H R O N R' R" R" R', R" can be H, alkyl, or aryl. Ch. 17 - 65 O O O + 2 NH3 Phthalamic anhydride O H2O NH2 warm O NH4 O NH2 OH H3O+ O Ammonium phthalamate (94%) (- NH4+) Phthalamic acid O (81%) Ch. 17 - 66 O O NH2 150-160oC N OH O Phthalamic acid + H2O H O Phthalimide (~ 100%) Ch. 17 - 67 8D. Amides from Esters O O H + R OR'" N R' R R" N R' + R'"OH R" R' and/or R" may be H. e.g. O O OMe MeNH2 N heat H Me + MeOH Ch. 17 - 68 8E. Amides from Carboxylic Acids and Ammonium Carboxylates O R O OH + NH3 R O NH4 heat O H2O + R NH2 Ch. 17 - 69 DCC-Promoted amide synthesis O R O 1. DCC OH 2. R'NH2 R N R' + DCU H Ch. 17 - 70 Mechanism C6H11 C C O: : + :N H O C6H11 C : H N N: : : C :O R : R N : : O: C6H11 C6H11 : Dicyclohexylcarbodiimide (DCC) R N O C : O : H : : C C6H11 N: C6H11 Ch. 17 - 71 Mechanism (Cont’d) C6H11 C proton R : transfer : : : : O C6H11 R' C NH2 NHC6H11 : NH2 reactive intermediate : C : : R N C NHC6H11 C6H11 O O C6H11 : N: C N : : O : C O: : H N : : R O : : : : O: R' + C an amide O C NHC6H11 : NHR' : : R NHC6H11 N,N'-Dicyclohexylurea (DCU) Ch. 17 - 72 8F. Hydrolysis of Amides Acid hydrolysis of amides O R O + H NH2 H2O, heat R OH + NH4 Ch. 17 - 73 Mechanism R NH2 O :O : OH H2O NH2 H R H NH2 : R O H+ : :O : H O H : OH : R OH R OH + NH3 R HO NH3 Ch. 17 - 74 Basic hydrolysis of amides O R O OH NH2 H2O, heat R O + NH3 Ch. 17 - 75 Mechanism O R OH NH2 O O R HO NH2 R O H + NH2 O NH3 + R O Ch. 17 - 76 8G. Nitriles from the Dehydration of Amides :O : P4O10 or (CH3CO)2O : R NH2 heat (H2O) N: + H3PO4 (or CH3CO2H) (a nitrile) R C This is a useful synthetic method for preparing nitriles that are not available by nucleophilic substitution reactions between alkyl halides and cyanide ions Ch. 17 - 77 e.g. O NH2 P4O10 C N dehydration Ch. 17 - 78 Example ● Synthesis of C N NaCN Br DMSO CN 1o alkyl bromide SN2 reaction with ⊖CN works fine Ch. 17 - 79 CN But synthesis of Br NaCN DMSO No Reaction! 3o alkyl bromide No SN2 reaction Ch. 17 - 80 Solution Br O 1. Mg, Et2O OH 2. CO2 3. H3O+ 1. SOCl2 2. NH3 O CN P4O10 NH2 dehydration Ch. 17 - 81 8H. Hydrolysis of Nitriles R C N O base or acid H2O, heat R OH Catalyzed by both acid and base Ch. 17 - 82 Examples CN OH H2SO4 H2O, O (82%) OH CN 1. NaOH, H2O, + 2. H3O O (68%) Ch. 17 - 83 Mechanism C N: H R NH C NH + : O H + :O C H NH : R NH : R NH2 O: C H H2O : O : H H H slow : amide tautomer H O: C C H : R O : H H R H : R protonated nitrile protonated amide H several steps C (amide hydrolysis) NH2 : R O: O R + OH NH4 Ch. 17 - 84 R H : HO OH H : : NH2 R OH OH OH H NH OH H O : NH2 O OH HO : : H HO HO O R H R OH O : : : H NH2 R : + O O NH N: O R OH : : C H N : R Mechanism : H O OH R O + NH3 + OH Ch. 17 - 85 8I. Lactams O O O NH H N O S N O g a g-lactam a -lactam R NH CH3 CH3 CO2H NH d g a d-lactam R = C6H5CH2 Penicillin G R = C6H5CH Ampicillin NH2 R = C6H5OCH2 Penicillin V Ch. 17 - 86 9. Derivatives of Carbonic Acid 9A. Alkyl Chloroformates and Carbamates (Urethanes) Alkyl chloroformate O ROH + Cl O Cl RO Cl + HCl alkyl chloroformate Ch. 17 - 87 e.g. OH O + RO Cl O HCl + O Cl Benzyl chloroformate Ch. 17 - 88 Carbamates or urethanes O RO O Cl + R'NH2 OH RO NHR' a carbamate (or urethane) Ch. 17 - 89 protected amine Protection O O R O NH2 + N OH R N O H Deprotection H2, Pd O R Cl R NH2 + CO2 + O H HBr, CH2CO2H R NH3 + CO2 + Br Ch. 17 - 90 10. Decarboxylation of Carboxylic Acids O R OH O R decarboxylation O R O o 100-150 C OH H + CO2 R + CO2 A -keto acid Ch. 17 - 91 There are two reasons for this ease of decarboxylation O H O R O CO2 -keto acid R ketone enol O : : O CO2 : : R O R O O H O: HA : R O : : R acylacetate ion : O : : R resonance-stabilized anion Ch. 17 - 92 11. Chemical Tests for Acyl Compounds Recall: acidity of O O O H O H H > > Ch. 17 - 93 O R O O H + Na OH R + H2O O Na (soluable in water) O H O Na + NaOH (soluble in water) O (immiscible with H2O) H + NaOH No Reaction Ch. 17 - 94 O O O H + NaHCO3 O Na + CO2(g) + H2O (gas evolved) O O H H + NaHCO3 No Reaction + NaHCO3 No Reaction Ch. 17 - 95 12. Polyesters and Polyamides: Step-Growth Polymers Polyesters HO m O OH + HO O n OH -H2O O O O m (a polyester) O n Ch. 17 - 96 Polyamides O H2N m N H + O n Cl Cl H -HCl H H N N O m (a polyamide) O n Ch. 17 - 97 Example: Nylon 66 O OH n HO NH2 + n H2N O heat H O N O (Nylon 66) ● + 2n H2O N H n Applications: clothing, fibers, bearings Ch. 17 - 98 Example: Dacron (Mylar) O O n CH3O OCH3 + OH n HO 200oC O O O + 2n CH3OH O (Dacron) ● n Applications: film, recording tape Ch. 17 - 99 13. Summary of the Reactions of Carboxylic Acids and Their Derivatives O Reactions of carboxylic acids O R C OH R 1. P, X2 2. H2O X 1. LiAlH4 R + O OH R'OH, H+, R O O C C O R' R' base Cl SOCl 2 or PCl3 or PCl5 C R 2. H2O, H O O NaOH or NaHCO3 or other bases O RCH2OH C OR' O R C Cl Ch. 17 - 100 Reactions of acyl chlorides O R O NR' R OH H2O R'2NH O R Cl R'OH, base O R R'COOH base O O R' O R OR' Ch. 17 - 101 Reactions of acyl chlorides (Cont’d) O R R 1. LiAlH4 benzene AlCl 3 R Cl 1. LiAlH(OtBu)3, -78oC 2. H3O+ O 1. R'MgX R' R' 2. H3O+ O R OH 2. H3O+ OH R H Ch. 17 - 102 Reactions of acid anhydrides O R O OH + HO R' H2O O R"2NH R O R O O R"OH R' O O NR" + R' O NR"2H2 R O OR" + HO R' Ch. 17 - 103 Reactions of esters O R OH H 1. DIBAL, -78oC 2. H3O+ 1. LiAlH4 2. H3O+ OH R R R" R R NH3 O NH2 R OH H2O, H+, O O 1. R"MgX 2. H3O+ R" O OR' 1. OH2. H2O, H+ R"OH, H+, R OH O R OR" Ch. 17 - 104 Reactions of nitriles R O NH2 R H+, H2O, 1. LiAlH 4 2. H3O+ R C N 1. LiAlH(O tBu)3 R OH, H2O, O or DIBAL, -78oC O 2. H3O+ H OH R O Ch. 17 - 105 Reactions of amides O R + HNR'2 OH H2O, H+ or OH- P4O10 (P2O5) or Ac2O, D (R' = H only) O R N R' 1. LiAlH4 2. H3O+ R' R C N R NR'2 Ch. 17 - 106 END OF CHAPTER 17 Ch. 17 - 107