Slide 1 - Center for Sustainable Energy
advertisement

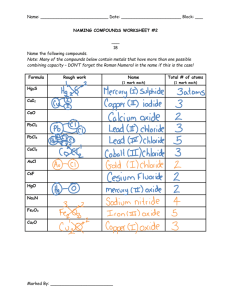
Solar Thermochemical Ammonia: A More Sustainable Way to Feed the World 1,2,3Brian Peterson, 2Ronny Michalsky, 2Peter Pfromm REU Program, 2Kansas Brian State University PetersonCenter for Sustainable Energy 3Missouri University of Science and Technology Department of Chemical Engineering 1NSF Introduction Ammonia is critical as a source of fixed nitrogen as fertilizer for agriculture globally. It has been estimated that between one third and one half of the world’s population could not be sustained without chemical processes that synthetically produce ammonia from nitrogen in the air. The current standard for ammonia production is the Haber-Bosch process. This 100-year-old process consumes large quantities of fossil fuels and requires large amounts of energy (accounts for nearly 2% of energy usage globally). It is imperative to study alternative, sustainable processes to feed both the growing world population and the growing biofuel industry. Reduction of Magnesium Oxide H 2O In general, the Solar Ammonia process utilizes a metal nitride which is corroded with a hydrogen source (either H2 or H2O). When water is used, the metal nitride corrodes very easily at room temperature and atmospheric pressure, but a rather unreactive metal oxide is formed. The more difficult step is recycling the metal oxide because it requires much higher temperatures and a reducing agent such as carbon. Tasks Reduction of Magnesium Oxide • Test effects of the addition of Iron(III) Oxide and Chromium(III) Oxide at 1200 °C • Analyze kinetics of best case mixtures Corrosion of Calcium Nitride Tab1: Percent of Magnesium atoms that left the system at different reaction times which correlates to Magnesium reduced. Mg Nitride +CO ← Mg Oxide + C +N2 N2 C (Biomass/Charcoal) Goal Molar Ratio Mass % Mg Lost MgO:C:FeO3:Cr2O3 30 min. 1:8:0:0 49.21% 1:8:1:0 -60.80% 1:8:0:1 7.09% Reduce Magnesium Oxide into the form of Magnesium Metal or Magnesium Nitride Methods Comparison Process Haber-Bosch Solar Ammonia Pressure 300 bar 1 bar Temperature 500 °C 400-1200 °C Energy Source Natural Gas Solar Energy Hydrogen Source Natural Gas Water or H3 Reaction Catalyst No Catalyst • Higher ratios of Carbon to Magnesium Oxide heavily influence Magnesium Nitride • Chromium(III) Oxide appears to have a stronger influence than Iron(III) Nitride on the production of Magnesium Nitride NH3. Mg Nitride + H2O → Mg Oxide + NH3 CO Corrosion of Calcium Nitride Conclusions from Figure 1: 60 min. 120 min. Fig1: Yield of 8 mixtures using Solar Concentrator 0.1 0.075 Magnesium Nitride Produced (g) per 50 mg Magnesium Oxide 0.05 0.025 Temp=1200 °C 0 • Identify composition of solids after reaction • Analyze the kinetics at various H2 flow rates Molar Ratio (MgO:C:Fe2O3:Cr2O3) NH3. Ca Nitride + H2 → “Ca solids”+ NH3 Ca Nitride ← “Ca solids” + N2 N2 Methods • React Calcium Nitride with H2 at various 50.55% -43.35% temperatures using an indoor reactor and determine the composition of the solids -74.01% -154.78% • Gather kinetics data for the reaction at 700°C -24.82% 44.06% • Make different molar combinations of 3 solid Conclusions from Table 1: reactants • Heavy error exists, most likely in the X-Ray • React Nitrogen using Solar Diffraction analysis of solid composition Concentrator and reactor. • Better analytical techniques might be • Gather data on kinetics of 3 considered mixtures, one with Iron(III) Oxide added, one with Fig3: Mass percent of Calcium Hydride in the Chromium (III) Oxide solid phase after reactions at various temperatures added, and a reference 25% without additional oxides. Percent of CaH2 in the Solid Phase Run using an indoor 20% Solar Concentrator furnace and reactor 15% Reaction Time=1 hr. 10% Results and Conclusions H2 5% Goal Maximize the production of Ammonia Results and Conclusions Fig2: Mol percent of Nitrogen atoms that left the system, presumably as Ammonia at different reaction temperatures 70% Percent N Liberated to Gas Phase 60% 50% Reaction Time=1 hr. 40% 30% 20% 0 500 1000 1500 Temperature (°C) Conclusions from Figure 2: 0% 1500 • As expected, more Ammonia is produced (Nitrogen liberated) at higher temperatures, but levels off after 700 °C Fig4: Mol percent of Nitrogen atoms liberated, • Flow rate experiments (Figure 4) should be performed at 700 °C likely as Ammonia at different Hydrogen flows 100% Conclusions from Figure 3: Percent N Liberated to the Gas Phase • More Calcium Hydride is produced at higher 80% temperatures • Also Calcium Hydride is vaporized at the 60% higher temperatures and exits the system Reaction Time=2 hr. 40% Temp=700 °C Conclusions from Figure 4: • Hydrogen flow rate has little effect on 20% Ammonia formation 0 0.5 1 1.5 2 2.5 • Thus this is likely a diffusion limited reaction Hydrogen Flow Rate (L/min.) 0 500 1000 Temperature (°C)