Week3
advertisement
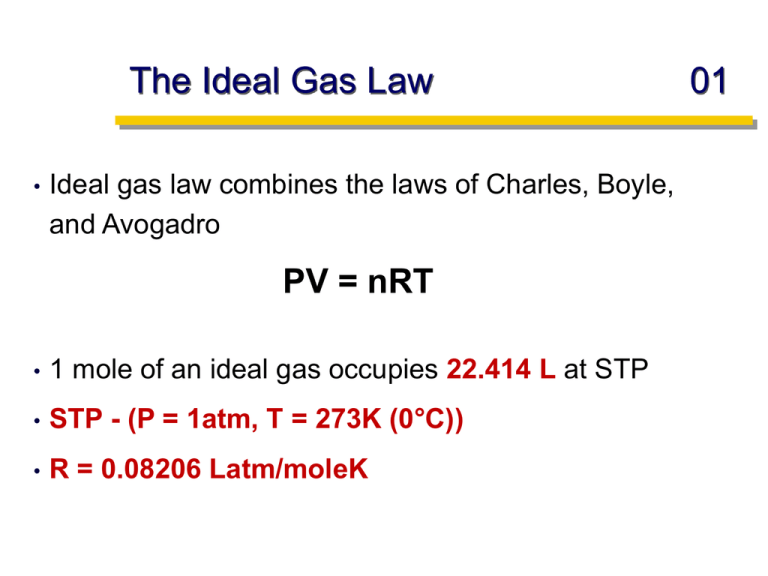
The Ideal Gas Law • Ideal gas law combines the laws of Charles, Boyle, and Avogadro PV = nRT • 1 mole of an ideal gas occupies 22.414 L at STP • STP - (P = 1atm, T = 273K (0°C)) • R = 0.08206 Latm/moleK 01 Example 02 Sulfur hexafluoride (SF6) is a colorless, odorless, very unreactive gas. Calculate the pressure (in atm) exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 L at 69.5°C. PV = nRT P = nRT/V = (1.82moles)(0.08206Lat/molK)(342.5K) = 9.42atm (5.43L) What is the volume (in liters) occupied by 7.40 g of CO2 at STP? PV = nRT V = nRT/P = (0.168moles)(0.08206Latm/molK)(273K) = 3.76L (1atm) 7.40g x 1mole/44g = 0.168moles CO2 The Ideal Gas Law Density and Molar Mass Calculations: mass nM PM d volume V R T PV = nRT n = PV/RT 03 Example 04 What is the molar mass of a gas with a density of 1.342 g/L at STP? PV = nRT n = PV/RT = (1atm)(1L) = 0.0446moles gas (0.08206Latm/moleK)(273K) 1.342g/0.0446moles = 30.1g/mol = 30.1 amu What is the density of uranium hexafluoride, UF6, (MM = 352 g/mol) under conditions of STP? PV = nRT n = PV/RT = (1atm)(1L) (0.08206Latm/moleK)(273K) 352g/mol x 0.0446moles = 15.70g/L = 0.0446moles gas Example 05 The density of a gaseous compound is 3.38 g/L at 40°C and 1.97 atm. What is its molar mass? PV = nRT n = PV/RT = (1.97atm)(1L) = 0.0767 moles (0.08206)(313K) 3.38g/0.0767mol = 44.07g/mol Gas Stoichiometry In gas stoichiometry at STP: volume moles At STP: 1L air 1L Xe 1L O2 x moles 06 Gas Stoichiometry - Airbag movie 07 2NaN3(s) + (O)(s) Na2O(s) + 3N2(g) (O) is an oxidant (undergoes reduction-gains electrons) 145g of NaN3. How many liters of N2 gas are produced at 30°C and 1.15atm? 145g x 1mole/65.0g = 2.23moles NaN3 2.23moles NaN3 x 3moles N2 = 3.35moles N2 2moles NaN3 At STP volume moles VN2 = 3.35moles x 22.4L/1mole = 75.04 L NOT at STP! V = nRT/P = (3.35mol)(0.08206Latm/molK)(303K) = 72.4L (1.15atm) Example 08 Hydrogen gas, H2, can be prepared by letting zinc metal react with aqueous HCl. How many liters of H2 can be prepared at 742 mm Hg and 15oC if 25.5 g of zinc (MM = 65.4 g/mol) was allowed to react? Zn(s) + 2 HCl(aq) H2(g) + ZnCl2(aq) 25.5g Zn x 1mole Zn x 1mole H2 = 0.390moles H2 65.39g Zn 1mole Zn V = nRT/P = (0.390mol)(0.08206Latm/molK)(288K) = 9.44L H2 742mm Hg x 1atm/760mm Hg Dalton’s Law of Partial Pressures 09 Air N2 + O2 + Ar + CO2 Ptotal (air) = PN2 + PO2 + PAr + PCO2 PN2 = partial pressure (pressure alone in container) In a mixture of gases (Air) the total pressure (Ptotal) is the sum of the partial pressures of the gases: RT P total V n Dalton’s Law of Partial Pressures 10 Partial pressure(N2) in air? RT P total V Concentration of each component: mole fraction (X) = n moles of component total moles of the mixture XN2 = nN2 = nN2 nN2 + nO2 + nAr + nCO2 n = PV/RT ntotal XN2 = PN2 (V/RT) = PN2 Ptotal (V/RT) Ptotal PN2 = XN2 Ptotal Example 11 Exactly 2.0 moles of Ne and 3.0 moles of Ar were placed in a 40.0 L container at 25oC. What are the partial pressures of each gas and the total pressure? ntotal = 2moles + 3moles = 5moles Ptotal = ntotalRT/V = (5moles)(0.08206Latm/molK)(298K) = 3.06atm 40.0L PNe = XNe Ptotal = (2moles / 5moles)(3.06atm) = 1.224atm PAr = XAr Ptotal = (3moles / 5moles)(3.06atm) = 1.836atm Example 12 A sample of natural gas contains 6.25 moles of methane (CH4), 0.500 moles of ethane (C2H6), and 0.100 moles of propane (C3H8). If the total pressure of the gas is 1.50 atm, what are the partial pressures of the gases? ntotal = 6.25mol + 0.500mol + 0.100mol = 6.85moles PCH4 = XCH4 Ptotal = (6.25/6.85)(1.5atm) = 1.369 PC2H6 = XC2H6 Ptotal = (0.500/6.85)(1.5atm) = 0.109 PC3H8 = XC3H8 Ptotal = (0.100/6.85)(1.5atm) = 0.022 Ptotal = 1.369 + 0.109 + 0.022 = 1.5atm Example 13 Hydrogen gas generated when calcium metal reacts with water is collected at 30°C and a pressure of 988 mm Hg. The volume collected is 641 ml. What is the mass (in grams) of the hydrogen gas obtained? The pressure of the water vapor at 30°C is 31.82 mm Hg. Ca(s) + H2O(l) CaO(s) + H2(g) Ptotal = PH2 + PH2O PH2 = Ptotal - PH2O = 988mm Hg - 31.82mm Hg = 956.18mm Hg n = PV/RT = (956.18mm Hg x 1atm/760mm Hg)(0.641L) = 0.0324 moles H2 (0.08206Latm/molK)(303K) 0.0324moles x 2g/1mole = 0.0648g = 64.8mg H2 Ideal versus nonideal gases 14 Some substances are ideal gases, while other substances are nonideal gases FALSE! All gases are ideal under certain conditions (STP) and nonideal under other conditions. Ideal gases 15 Assumptions: • Volumes of the particles themselves are negligible compared with the total volume of the gas • There are no attractive or repulsive forces between particles Behavior of Real Gases 16 Volume of the particles are not negligible! The volume taken up by gas particles is more important at higher pressures than at lower pressures. As a result, the volume at high pressure will be greater than the ideal value. Behavior of Real Gases 17 At higher pressures, particles are much closer together and attractive forces become more important than at lower pressures. Molecules are drawn closer together, decreasing the volume. Ideal gas law will over shoot the volume of the gas.