Molar Volume of a Gas Calculations Daltons Law Mixed Gas Law
advertisement

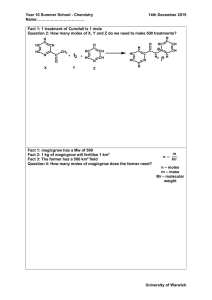
Read the Lab Procedure. What is the objective of today’s lab in your own words? On a phase diagram, what do the “plateaus” indicate? What do the slopes indicate? Review Graham’s WS Review ▪ Calculate the molar volume of a gas using experimental data ▪ Demonstrate my knowledge of gas laws by completing an assessment. Pass fwd Practice Problems Lab You will have 15 minutes during lab to work on HW – gas law problems Reminder: Test on Wed or Th Wear goggles and aprons Working with 3 M HCl – wash with water for 5 minutes if you get on skin Liquids can be put down the drain Demo – need to be very careful this lab. Any horsing around, you will be removed!! 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Brooke, Mira Sophia, Brendan Maddie, Emily Adam, Niijae Garrett, Harper Kristine, Devin Meghan, Ergi Eli, Alayna Clay, Thomas Michael, Paulos Sophia, Cade Travis, Maria 1. What is the mass of the magnesium strip you used? 2. How many moles of magnesium does this represent? 1. How can we complete this conversion? 3. How many moles of hydrogen should be produced in the reaction? ▪ ▪ ▪ Note: We are assuming all of the Magnesium was consumed Hint: What is the balanced equation for the reaction that we ran in lab? Using that information how could we determine the number of moles of hydrogen? 4. What is the vapor pressure of water under the conditions of this experiment? ▪ ▪ Vapor pressure can be looked up using a table of values at a given temperature. PH2O = 20.0 mmHg 5. What is the pressure of JUST hydrogen gas? ▪ Ptotal = PH2O + PH2 6. What would the volume of this gas be at STP? Use the combined gas law What was the pressure of H2, volume of H2 and temperature in the experiment? What are the values of STP? 7. What is the volume of one mole of this gas at STP? ▪ Based on what we have calculated already, how can we determine this? Note: The data table on the back of the lab is a place to fill in your numbers from the front of the lab.