Ions in Chemical Compounds (Criss-cross chart)
advertisement

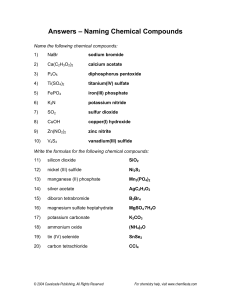
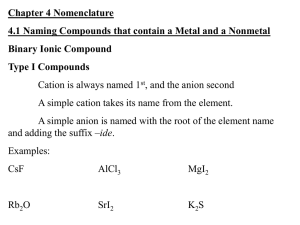
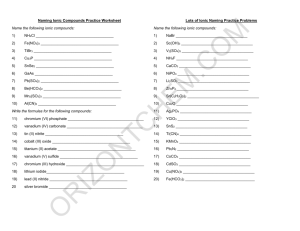
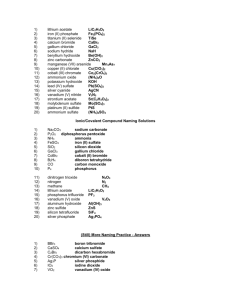
Ions in Chemical Compounds Name______________________________ Complete the following table, being sure that the total charge on the resulting compound is zero. Determine the charges for each ion and cross them to make compounds. Ions Sodium Na Ammonium NH4 Potassium K Calcium Ca Magnesium Mg Aluminum Al Iron (II) Fe Iron (III) Fe Copper (I) Cu Chloride Hydroxide Cl OH Nitrate NO3 Sulfide Carbonate Phosphate S CO3 PO4 Naming Ionic Compounds Give the name and molar mass of the following ionic compounds: Name 1) Na2CO3 ______________________________________________________________ 2) NaOH _______________________________________________________________ 3) MgBr2 _______________________________________________________________ 4) KCl _________________________________________________________________ 5) FeCl2 ________________________________________________________________ 6) FeCl3 ________________________________________________________________ 7) Zn(OH)2 _____________________________________________________________ 8) Be2SO4 ______________________________________________________________ 9) CrF2 ________________________________________________________________ 10) Al2S3 ________________________________________________________________ 11) PbO ________________________________________________________________ 12) Li3PO4 _______________________________________________________________ 13) TiI4 _________________________________________________________________ 14) Co3N2 _______________________________________________________________ 15) Mg3P2 _______________________________________________________________ 16) Ga(NO2)3 ____________________________________________________________ 17) Ag2SO3 ______________________________________________________________ 18) NH4OH ______________________________________________________________ 19) Al(CN)3 _______________________________________________________________ 20) Be(C2 H3O2)2 __________________________________________________________ For the following compounds, give the formulas and the molar masses: Formula 22) sodium phosphide _____________________________________________________ 23) magnesium nitrate _____________________________________________________ 24) lead (II) sulfite _________________________________________________________ 25) calcium phosphate _____________________________________________________ 26) ammonium sulfate _____________________________________________________ 27) silver cyanide _________________________________________________________ 28) aluminum sulfide ______________________________________________________ 29) beryllium chloride ______________________________________________________ 30) copper (I) arsenide _____________________________________________________ 31) iron (III) oxide _________________________________________________________ 32) gallium nitride _________________________________________________________ 33) iron (II) bromide _______________________________________________________ 34) vanadium (V) phosphate ________________________________________________ 35) calcium oxide __________________________________________________________ 36) magnesium acetate _____________________________________________________ 37) aluminum sulfate _______________________________________________________ 38) copper (I) carbonate _____________________________________________________ 39) barium oxide __________________________________________________________ 40) ammonium sulfite _______________________________________________________ 41) silver bromide __________________________________________________________ 42) lead (IV) nitrite _________________________________________________________ Naming Covalent Compounds worksheet Write the formulas for the following covalent compounds: 1) antimony tribromide __________________________________ 2) hexaboron silicide __________________________________ 3) chlorine dioxide __________________________________ 4) hydrogen iodide __________________________________ 5) iodine pentafluoride __________________________________ 6) dinitrogen trioxide __________________________________ 7) ammonia __________________________________ 8) phosphorus triiodide __________________________________ Write the names for the following covalent compounds: 9) P4O5__________________________________ 10) O2 __________________________________ 11) SeF6 __________________________________ 12) Si2Br6 __________________________________ 13) SCl4 __________________________________ 14) CH4 __________________________________ 15) B2C __________________________________ 16) NF3 __________________________________ 17) H2O _________________________________________ 18) PCl5 _________________________________________ 19) NO __________________________________________ 20) PI3 _________________________________________ Chemistry: Errors in Chemical Formulas and Nomenclature Each of the following formulas or chemical names contains an error. Correct each example. 1. aluminum (III) iodide 1. ______________________ 2. Al3O2 2. ______________________ 3. (NH4)3Cl2 3. ______________________ 4. lead (III) oxide 4. ______________________ 5. leadic oxide 5. ______________________ 6. stannous (II) bromide 6. ______________________ 7. monocalcium dioxide 7. ______________________ 8. K(ClO2) 8. ______________________ 9. (OH)3Al 9. ______________________ 10. Cr4(CN)3 10. ______________________ 11. Pb(NO3)3 11. ______________________ 12. copper (I) chloric 12. ______________________ 13. ferric (III) oxide 13. ______________________ 14. NiNO32 14. ______________________ 15. Mg2F 15. ______________________ 16. ironic oxide 16. ______________________ 17. ClK 17. ______________________ 18. Ca2O2 18. ______________________ 19. sodium (I) fluoride 19. ______________________ 20. dititanium tetroxide 20. ______________________ 21. dihydrogen oxide 21. ______________________ 22. magnesium dihydroxide 22. ______________________ 23. cuprous (I) sulfate 23. ______________________ 24. strontium difluoride 24. ______________________ 25. NO3Na 25. ______________________ Review– Naming Chemical Compounds The following are a good mix of naming and formula writing problems to help you get some practice. Name the following chemical compounds: 1) NaBr ________________________________________________________________ 2) Ca(C2H3O2)2 __________________________________________________________ 3) P2O5 _________________________________________________________________ 4) Ti(SO4)2 ______________________________________________________________ 5) FePO4 _______________________________________________________________ 6) K3N __________________________________________________________________ 7) SO2 __________________________________________________________________ 8) CuOH ________________________________________________________________ 9) Zn(NO2)2 ______________________________________________________________ 10) V2S3 __________________________________________________________________ Write the formulas for the following chemical compounds: 11) silicon dioxide __________________________________________________________ 12) nickel (III) sulfide ________________________________________________________ 13) manganese (II) phosphate _________________________________________________ 14) silver acetate ____________________________________________________________ 15) diboron tetrabromide ______________________________________________________ 16) magnesium sulfate heptahydrate _____________________________________________ 17) potassium carbonate ______________________________________________________ 18) ammonium oxide _________________________________________________________ 19) tin (IV) selenide __________________________________________________________ 20) carbon tetrachloride _______________________________________________________ Name _________________________________________________________________ Date ______________ Class ______ Naming Acids Acids are molecular compounds that dissolve in water to produce hydrogen ions (H +). Although acids are molecular compounds they act like ionic compound in water because they separate into a cation and an anion. The cation is always H +, and the anion depends on the particular acid. An acid is named for the characteristic anion it produces. Below is a flow chart illustrating the rules for naming acids. If the anion ends in… -ide add the hydro- prefix replace –ide with –ic and add the word acid Example: HCl inonizes into H+ & ClCl- = chloride Hydrochloric Acid -ate -ite replace –ate with –ic and add the word acid replace –ite with -ous and add the word acid DO NOT ADD THE PREFIX HYDRO- DO NOT ADD THE PREFIX HYDRO- Example: HC2H3O2 ionizes into H+ & C2H3O2C2H3O2- = acetate Acetic Acid Example: HNO2 ionizes into H+ & NO2NO2- = Nitrite Nitrous Acid Anions - 1 Acetate…….….C2H3O2 Hydroxide…….…..OHAluminate……..AlO2Iodate……………...IO3Amide………….NH2 Iodite…………......IO2Astatate…….......AtO3 Nitrate…………….NO3Azide…………….N3Bicarbonate…….HCO3- Nitrite…………...NO2Perbromate…….…BrO4Bismuthate….....BiO3Perchlorate………..ClO4Bromate……….BrO3 Periodate…………..IO4Chlorate…….….ClO3 Permangnate….…MnO4Chlorite………...ClO2 Chromite….….….CrO2- Perrhennate…….....ReO4Thiocyanate………..SCNCyanate………. CNOVanadate…………...VO3 Cyanide………..CNDihydrogen phosphate…H2PO4Formate………………….HCO2Hypochlorite…………..…..ClOHypoiodite… ………………IOHydrogen sulfite…..…….HSO3Hydrogen sulfate………..HSO4Hydrogen carbonate…….HCO3- - - 2 Carbonate…………….CO3 Carbonite…………….CO22Chromate………….….CrO42Dichromate………..…Cr2O72Hydrogen phosphate.HPO42Hydrogen phosphite.HPO32Hyposulfite ……….….SO22Oxalate…………….…C2O42Molybdate……….…MoO42Peroxide ………..……..O22Selenate………….…SeO32Silicate……………….SiO32Sulfate……………..…SO42Sulfite……………..….SO32Tartrate……………C4H4O62Tellurate………..…..TeO42Tellurite……………..TeO32Tetraborate……….....B4O72Thiosulfate…………..S2O32Tungstate…………….WO422- 3 Aresenate……AsO43Aresenite……AsO33Borate…………BO33Citrate….….C6H5O73Phosphate….....PO43Phosphite…..…PO33Hypophosphite...PO23- - 4 Pyrophosphate……P2O74- Name the following Acids: HClO ______________________________________________________________ HCN ______________________________________________________________ H2SO3 ______________________________________________________________ H2SiO3 _______________________________________________________________ H3BO3 _______________________________________________________________ H2CO3 _______________________________________________________________ HF _______________________________________________________________ HClO4 _______________________________________________________________ HNO3 _______________________________________________________________ HNO2 _______________________________________________________________ H2CrO4 _______________________________________________________________ H3PO4 ________________________________________________________________ HI ________________________________________________________________ Write a formula for each of the following acids: Nitric Acid Hydrocyanic Acid Chloric Acid Acetic Acid Sulfurous acid Hydrobromic Acid Sulfurous Acid Chlorous Acid Hydrochloric Acid Phosphoric Nitrous Acid Hydroiodic Acid Hydrofluoric Acid Phosphorous Acid Hydrosulfuric acid Carbonic Acid Sulfuric Acid