Stoichiometry Calculations with Chemical Formulas and Equations
advertisement

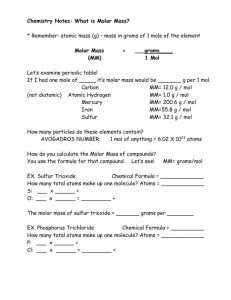
Stoichiometry Calculations with Chemical Formulas and Equations Chapter 3 BLB 12th Expectations Balance chemical equations. g ↔ moles ↔ molecules ↔ atoms Find empirical and molecular problems. Calculate amounts of reactants and products. Calculate theoretical and percent yield. Stoichiometry Quantity relationships based on chemical equations 3 Main Concepts: 1. Chemical formula – molar ratio of atoms 2. Chemical equations – molar ratio of compounds 3. Law of Conservation of Mass: mass of reactants = mass of products 3.1 Chemical Equations Components: reactants → products Physical states (s, l, g, aq) Reaction conditions (heat Δ, light, solvents, etc.) Coefficients determine molar ratios. The number of moles of each type of atom must be the same on each side. Balancing: By inspection Use coefficients; don’t change chemical formulas Coefficients vs. Subscripts Fe2S3(s) + HCl(aq) KClO3(s) HNO3(l) + → P4O10(s) → FeCl3(s) KCl(s) → + + H2S(g) O2(g) (HPO3)3(l) + N2O5(g) 3.2 Some Simple Patterns of Chemical Reactivity Combination and Decomposition combination: A + B → C 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) decomposition: C → A + B 2 NaN3(s) → 2 Na(s) + 3 N2(g) 3.2 Some Simple Patterns of Chemical Reactivity Combustion burning of a fuel in the presence of oxygen products of complete combustion: CO2, H2O exothermic (produces heat) Molecular view of methane combustion 3.2 Some Simple Patterns of Chemical Reactivity Combustion C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) 2 CH3OH(g) + 3 O2(g) → 2 CO2(g) + 4 H2O(g) Each C atom in fuel produces 1 mol CO2 Each H atom in fuel produces ½ mol H2O 3.3 Formula Weights Formula and Molecular Weights (amu) formula weight – general molecular weight – molecules formula unit weight – ionic compound - sum of the atomic masses of each atom in chemical formula % Composition part percent 100% whole % composition by mass Mass of one type of atoms over mass of all atoms 3.4 Avogadro’s Number and the Mole Word association: pair – dozen – case – ream – 3.4 Avogadro’s Number and the Mole amu impractical for lab use (too small) Avogadro’s number: 6.0221421 x 1023 mol-1 The number of atoms in exactly 12 g of 12C For conversions: 6.022 x 1023 ?/mol, where ? can equal atoms, molecules, ions, etc. 1 mole = Avogadro’s number of anything molar mass – mass in grams of one mole of a substance, which is equal to the atomic mass in amu; g/mol 3.4 Avogadro’s Number and the Mole Atoms & compounds have different masses, thus the mass of 1 mole of atoms & compounds are different. 3.4 Avogadro’s Number and the Mole Conversions: g → mol divide by molar mass mol → g multiply by molar mass Abbreviations: mole – mol molarity – M Practice with Avogadro’s # & the Mole 3.5 Empirical Formulas from Analyses Empirical formula – smallest whole number ratio of atoms Molecular formula – actual ratio of atoms in a compound; multiple of the empirical formula; must know molar mass of compound Use % composition to find formula Problems A once-used gasoline additive contains 49.5% C, 3.2% H, 22.0% O, and 25.2% Mn. Determine the emipirical formula of this compound. Azulene, a hydrocarbon, contains 93.71% C. Its molar mass is ~128 g/mol. Determine the emipirical and molecular formulas for azulene. 3.5 Empirical Formulas from Analyses Summary: % data → grams Grams → moles Moles → molar ratio → empirical formula Empirical formula → molecular formula 3.5 Empirical Formulas from Analyses Combustion analysis 1 mol C in fuel → 1 mol CO2 2 mol H in fuel → 1 mol H2O Problems The combustion of propane, a hydrocarbon, produces 2.641 g CO2 and 1.442 g H2O. Determine the emipirical formula of propane. 3.6 Quantitative Information from Balanced Equations 3 Main Concepts: 1. Chemical formula – molar ratio of atoms 2. Chemical equations – molar ratio of compounds 3. Law of Conservation of Mass: mass of reactants = mass of products What’s balanced in a balanced equation? Stoichiometry Problems Use these 4 steps as a guide: (p. 97) Write & balance chemical equation. Convert to moles. Apply molar ratio. Convert from moles to quantity desired (mass, volume, etc.) Stoichiometry Problems How many grams of CaCl2 is produced from taking 2 antacid tablets, each containing 500. mg of CaCO3? CaCO3(s) + 2 HCl(aq) → CO2(g) + H2O(l) + CaCl2(s) From Sample Exercise 3.16, p. 98 How many grams of HCl are needed to react with 1000 mg of CaCO3? CaCO3(s) + 2 HCl(aq) → CO2(g) + H2O(l) + CaCl2(s) 3.7 Limiting Reactants Limiting reactant – reactant that is completely consumed; limits the amount of product that can be formed Theoretical yield – calculated yield of a product based on limiting reactant Percent yield experimental yield % yield 100% theoretical yield 78. Calculate the theoretical yield (in grams) of NO when 2.00 g of NH3 react with 2.50 g of O2. NH3(g) + O2(g) → NO(g) + H2O(g) Silver metal reacts with elemental sulfur according to the reaction below. If 2.0 g each of silver and sulfur react, what is the theoretical yield (in grams) of silver(I) sulfide? How many grams are left over? 16 Ag(s) + S8(s) → 8 Ag2S(s) How many grams are left over? 16 Ag(s) + S8(s) → 8 Ag2S(s) 84. When hydrogen sulfide gas is bubbled into a solution of sodium hydroxide, the reaction forms sodium sulfide and water. How many grams of sodium sulfide are formed if 1.25 g of hydrogen sulfide is bubbled into a solution containing 2.00 g of sodium hydroxide, assuming that the sodium sulfide is made in 92.0% yield? When 0.750 g iron(III) chloride hydrate is heated, 0.300 g of steam is produced. What is the value of x ? Δ FeCl (s) + x H O(g) FeCl3·x H2O(s) → 3 2