Organic Chemistry
advertisement

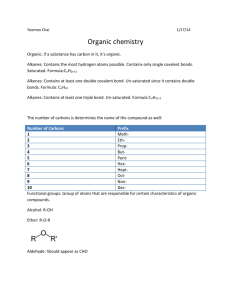
Organic Chemistry Template from: PresenterMedia.com Organic Compounds Organic compounds are molecules that contain carbon. • • Carbon is the base of organic compounds for 3 reasons: 1. Carbon forms stronger covalent bonds than most other non-metals 2. Carbon forms more stable molecules than most other non-metals 3. Carbon can form up to 4 bonds at a time, which gives many possibilities for molecules. Structure of Organic Compounds Besides carbon, the most common elements in organic compounds are: H, O, N, S, & halogens. • • • Organic compounds are held together with covalent bonds. Organic compounds must meet the octet rule (S does not break the octet rule) Hydrocarbons • Hydrocarbons are the most simple organic compounds • Hydrocarbons only contain C & H • All other organic compounds are derivatives of hydrocarbons • • Petrochemicals contain hydrocarbons, including: propane, butane, and octane Saturated – a compound is termed “saturated” if it has the maximum hybridization (sp3) at each carbon! Therefore: no double or triple bonds Hydrocarbons C2H6 Alkanes • Single bonded carbons C6H12 C2H2 Alkynes cycloalkane • A triple • ring bonded carbon C2H4 Alkenes A double bonded carbon • • CnH2n Strongest, • Weaker, less • Weakest, stable least stable most stable hydrocarbons hydrocarbo hydrocarbons • ns • • CnH2n+2 saturated • • CnH2n • • unsaturated CnH2n-2 unsaturated • unsaturated C6H6 Aromatics Ring of carbons with alternating double and single bonds Simple Organic Nomenclature Prefixes used with organic compounds 1. meth6. hex2. eth7. hept3. prop8. oct4. but9. non5. pent10. dec- Suffixes used with organic compounds -ane = CnH2n+2 -ene = CnH2n -yne = CnH2n-2 Name the following organic compounds a. CH4 b. C3H8 c. C3H6 d. C4H10 e. C5H8 f. C3H4 methane ___________________ propane ___________________ propene ___________________ butane ___________________ pentyne ___________________ propyne ___________________ Nomenclature Rules Procedure for naming carbon chains containing branches or substituents (non-straight chain) 1) Find the longest continuous carbon chain in the structure-this determines the root name 2) Any carbon not on this continuous chain is a substituent (appendage) 3) Number the main chain starting from the end closest to the first substituent 4) The substituents are still named according to the number of carbons (the suffix for a substituent is –yl instead of –ane) -CH3 methyl -CH2CH3 ethyl 5) Place all substituent names before the root name in alphabetical order 6) The substituent must be numbered to indicate the point of attachment to the main chain 7) Group multiple substituents of the same kind together and label di-, tri, etc. 8) When alphabetizing, the prefixes di-, tri- are ignored 9) With a ring compound the number of carbons in the ring determines the root name with a cyclo- prefix 10) Halogens are named as substituents with an -o suffix e.g. fluoro-, chloro-, bromo- or iodo- Isomers - Compounds with the same molecular formula but different structural arrangement therefore a different name. C6H14 A. B. C. D. hexane 2-methylpentane 3-methylpentane 2, 2-dimethylbutane E. 2,3-dimethylbutane Draw & Name the following 1-Butene H H H C C C H H H H H H C C C H H H H C C4H8 H 3-Heptyne H C7H12 H H C C H H C C H C3H6 or CH3CH=CH2 H H H 1-propene H C C C H H C4H8 or H2C=CHCH2CH3 1-Butene H C H H H C C C H H H H Functional Groups Functional groups are any atom, group of atoms, or organization of bonds that determine the properties of a molecule. • • • Functional groups are the most reactive part of a molecule Functional groups directly determine the properties of a molecule Functional Groups a. Alcohols: -OH Add –ol to name When carbon is single bounded to an –OH Ex. Propanol H 3-pentanol •• O •• H H H C C C H H H H b. Aldehydes: -C=OH - always on the end Example: Add –al to name pentanal c. Carboxylic Acids: COOH Most common organic acids belong to this group. Add –oic Acid to name Example: Propanoic Acid d. Ketones: -C=O (also called a carbonyl group) Example: Add –one to name 4 -octanone e. Esters are formed when a carboxylic acid reacts with an alcohol. This reaction is called esterification. Add –oate to name –O-C=O Example: methyl butanoate Smells like pineapple f. Halohydrocarbons: Halogen substituted for a hydrogen Example: 2-bromo-2-chloro-1,1,1-trifluoroethane. •• •• F •• • • •••• • • F • •Br• • C C •• F •• ••Cl •• •• •• H Polymers Polymers are long carbon chains made of repeating units • • Both natural and synthetic (man-made) Natural Polymer Examples •Nucleic • • Acids Examples: DNA, RNA Carbohydrates • Examples: starches, sugars • Example: lactase • Enzymes (protein catalysts that speed up chemical reactions) Vitamins (naturally in plants & animals) • Insulin (naturally made in your body) • Synthetic Polymer Examples • Plastics (made from petrochemicals) • Examples: polystyrene, Styrofoam, polyvinyl chloride (PVC), nylon • Kevlar (used in ballistic • Pharmaceuticals vests and combat helmets) • First synthetic pharmaceutical was aspirin