Orbital Diagrams & Electron Configuration Chemistry
advertisement
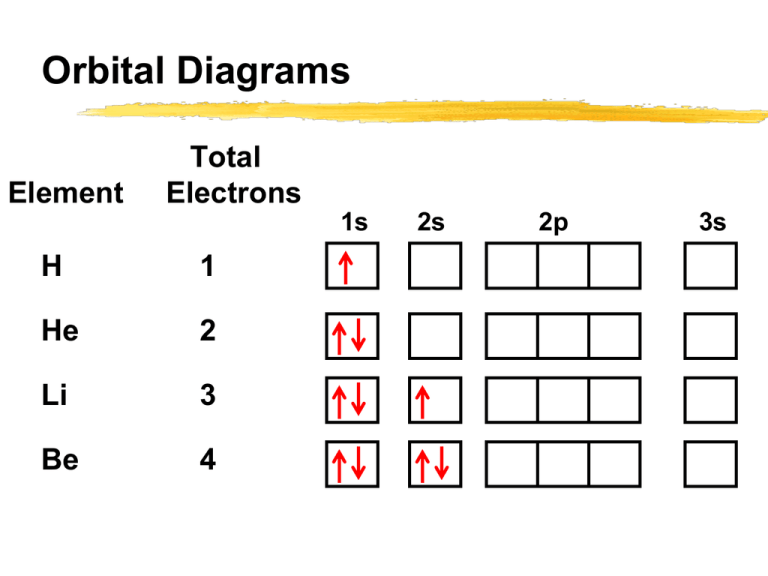
Orbital Diagrams Element Total Electrons 1s H 1 He 2 Li 3 Be 4 2s 2p 3s Orbital Diagrams Element Total Electrons 1s B 5 C 6 N 7 O 8 F 9 2s 2p 3s Orbital Diagrams For Ne (10 e-) 1s 2s 2p 3s Filling of the 2p subshell is complete at neon. The outermost shell (n = 2) contains an octet (8) of electrons. Orbital Diagrams Every noble gas has a complete outer shell. He: 2 electrons in the outer shell 1s2 All other noble gases : Ne, Ar, Kr, Xe, Rn an octet of electrons in the outer shell s2 p6 Orbital Diagrams This configuration is exceptionally stable. Responsible for the unreactive nature of the noble gases. Elements that ionize easily do so in a way that gives them the same octet of electrons: They become “isoelectric” with a noble gas! Orbital Diagrams For Sodium (Na) 11 electrons 1 more electron than the noble gas neon Valence e1s 2s 2p Neon core 3s highest energy level e- Orbital Diagrams Electrons that are in shells that are not occupied by the nearest noble gas element are called valence electrons. For Na, the 3s electrons are valence electrons Valence electrons: Used to form chemical bonds Orbital Diagrams Example: Draw the orbital diagram for potassium. How many valence electrons? Know: Z = 19 so there are 19 electrons 1s 2s 2p 3s 3p 4s Orbital Diagrams Example: Draw the orbital diagram for Ti (Z=22). How many valence electrons? 1s 2s 2p 3s 3d 3p 4s Electron Configuration Drawing orbital diagrams gives information not only about the orbitals that are/have been filled but also about the number of unpaired electrons. Orbital diagrams can be cumbersome!! Electron Configuration A short-hand notation is commonly used in place of orbital diagrams to describe the electron configuration of an atom. Electron configuration: a particular arrangement of electrons in the orbitals of an atom Electron Configuration The electron configuration tells the number of electrons found in each subshell. If there are three electrons in a 2p subshell, we would write: 2p3 where the superscript (3) indicates the number of electrons in that subshell Electron Configuration The orbital diagram for an O atom: 1s 2s 2p 3s The electron configuration for an O atom: 1s22s22p4 Electron Configuration To determine the electron configuration of an atom (or ion) without first writing the orbital diagram: determine the number of electrons present add electrons to each subshell in the correct filling order until all electrons have been added use the “diagonal” diagram to help determine the filling order Electron Configuration Example: Write the electron configuration of a Mn atom (Z = 25). How many valence e-? 1s22s22p63s23p64s23d5 Electron Configuration Example: Write the electron configuration of an O2- ion (Z = 8). With what noble gas is this ion “isoelectric”? An O2- ion has 8 protons and 10 electrons 1s22s22p6 Electron Configuration For Sodium (Na) 11 electrons 1 more electron than the noble gas neon 1s2 2s2 2p6 3s1 Neon core ABBREVIATED or CORE notation [Ne] 3s1 Electron Configuration Write the full electron configuration for F Then write the abbreviated or core notation F Electron Configuration Write the full electron configuration for Rb Then write the abbreviated or core notation Rb Electron Configuration Write the full electron configuration for Au Then write the abbreviated or core notation Au Electron Configuration Write the abbreviated or core notation Es Electron Configuration To write the electron configuration using core notation: find the noble gas that comes before the atom determine how many additional electrons must be added beyond what the noble gas has Atomic number of atom minus atomic number of noble gas Electron Configuration To write the electron configuration using core notation (cont): determine the period number of the element this determines the value of n of the s subshell to start with when adding extra electrons add electrons starting in the “n” s subshell Electron Configuration Example: Write the core electron configuration of Sr (Z = 38). Previous noble gas: Kr (Z = 36) Extra electrons: 38 - 36 = 2 Period number: 5 [Kr] 5s2 Electron Configuration Example: Write the core electron configuration of Br (Z = 35). Previous noble gas: Ar (Z = 18) Extra electrons: 35 - 18 = 17 Period number: 4 [Ar] 4s23d104p5 Orbital Diagrams Another useful periodic trend: p block d block f block Electron Configuration - Anomalies Some irregularities occur when there are enough electrons to halffill s and d orbitals on a given row. Electron Configuration - Anomalies For instance, the electron configuration for chromium is [Ar] 4s1 3d5 rather than the expected [Ar] 4s2 3d4. Isoelectronic Series When atoms ionize, they form ions with the same number of electrons as the nearest (in atomic number) noble gas. Na = 1s22s22p63s1 = [Ne]3s1 Na+ = 1s22s22p6 = [Ne] Cl = 1s22s22p63s23p5 = [Ne]3s23p5 Cl- = 1s22s22p63s23p6 = [Ar] Isoelectronic Series N (7 e-): N3- (10 e-): 1s22s22p3 1s22s22p6 = [Ne] O (8 e-): O2- (10 e-): 1s22s22p4 1s22s22p6 = [Ne] F (9 e-): 1s22s22p5 F- (10 e-): 1s22s22p6 = [Ne] Isoelectronic Series Na (11 e-): 1s22s22p63s1 Na+ (10 e-): 1s22s22p6 = [Ne] Mg (12 e-): 1s22s22p63s2 Mg2+ (10 e-): 1s22s22p6 = [Ne] Al (13 e-): Al3+ (10 e-): 1s22s22p63s23p1 1s22s22p6 = [Ne] 1A H Ions of the highlighted elements are isoelectronic with Ne. 2A Li Be Na Mg K Rb 8A 3A B 3B 4B 5B 6B 7B 8B Ca Sc Ti Sr Y Zr V Cr Mn Fe 8B 8B 1B 2B Co Ni 4A 5A 6A 7A He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Cs Ba La Hf Ta W Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Re Os Ir Pt Au Hg Tl Pb Bi Xe Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Isoelectronic Series Isoelectronic: having the same number of electrons N3-, O2-, F-, Ne, Na+, Mg2+, and Al3+ form an isoelectronic series. A group of atoms or ions that all contain the same number of electrons Isoelectronic Series Examples of isoelectronic series: N3-, O2-, F-, Ne, Na+, Mg2+, Al3+ Se2-, Br-, Kr, Rb+, Sr2+, Y3+ Cr, Fe2+, and Co3+ Periodic Properties of Elements Chemical and physical properties of the elements vary with their position in the periodic table. Atomic size Ionization energy Electronegativity Metallic character Periodic Properties--Atomic Size The relative size (radius) of an atom of an element can be predicted by its position in the periodic table. Trends Within a group (column), the atomic radius tends to increase from top to bottom Within a period (row), the atomic radius tends to decrease as we move from left to right Periodic Table Increasing size Periodic Properties--Atomic Size Increasing size Lower “lefter” larger Periodic Properties--Atomic Size Example: Which element would have the larger atomic radius, Ar or Br? Br should have the larger radius more towards the bottom more towards the left side Periodic Properties Ionization Energy The ease with which an electron can be removed from an atom to form an ion is an important indicator of its chemical behavior. Ionization energy: the minimum energy required to remove an electron from the ground state of an isolated gaseous atom or ion. Formation of cation (+) or more positively charged cation Periodic Properties Ionization Energy Ionization of Gaseous Sodium: Na (g) Na+ (g) + e As the ionization energy increases, it becomes harder to remove an electron. Periodic Properties Ionization Energy Within each row, the ionization energy increases from left to right Its easiest to remove an electron from an alkali metal and hardest to remove one from a noble gas. Within each group, the ionization energy generally decreases from top to bottom It’s easier to ionize K than Li. Periodic Properties Ionization Energy Example: Which element has the higher ionization energy, Br or Ca? Which one will lose an electron easier? Br has the higher ionization energy further to the right Ca will lose an electron easier because its ionization energy is lower. Periodic Properties Electronegativity The ability of an atom to attract electrons in a shared bond is called the electronegativity. Cl (g) + e- Cl- (g) The electron negativity increases as the attraction between an atom and an electron increases more electronegativity = more likely to pull an electron towards its nucleus Periodic Properties Electronegativity Trends: Halogens have the highest electronegativity Electronegativities increase moving from the left toward the halogens. Electronegativity decreases moving down a column. Noble gases have NO electronegativity Periodic Properties Electronegativity Trends: Noble gases will not accept another electron. To do so would require adding an electron to a new electron shell (significantly higher in energy) Halogens have highest electronegativity. Almost full valence shell Metals have low electronegativity; metals tend to lose electrons to form positive ions. Closer to nucleus: higher electronegativity Periodic Properties Metallic Character Metals: shiny luster malleable and ductile good conductors of heat and electricity form cations Metallic character increases from top to bottom Increases from right to left







