Chem Final Study Guide Energy How much heat energy must be
advertisement

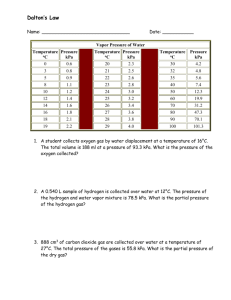
Chem Final Study Guide Energy 1) How much heat energy must be absorbed to completely vaporize 50.0 grams of H2O(l) at 100°C? a) Q = mHv = 50g(2260J/g) = 113,000J 2) Draw a heating and cooling graph. Label the states of matter at each section. Label the correct heat energy equation for each section of the graph. Label the phase change sections. Phase Changes 3) Draw a phase change diagram and label the triple point and critical point. Label solid, liquid, and gas. 4) Which phase changes are exothermic? a) Condensation, freezing, deposition 5) Which phase changes are endothermic? a) Melting, vaporization, sublimation Gases 6) What is the total pressure for a mixture of gases that contains four gases with partial pressures of 15.00 kPa, 4.56 kPa, 8.02 kPa, and 3.20 kPa. a) Pt = P1 + P2 + … = 15.00 kPa+ 4.56 kPa + 8.02 kPa + 3.20 kPa. = 30.78 kPa 7) The following data were recorded during experiments with a fixed amount of neon gas. Assuming that the gas was ideal, how many moles were there of the gas? Pressure (atm) Volume (L) Temperature (K) 5.6 35.0 320 a) PV=nRT 5.6atm(35L) = n(0.0821Latm/molK)(320K) 196 atmL = n26.272Latm/mol 7.46mol=n 8) A gas occupies a volume of 484 mL at 293 K and 99.0 kPa. What is the final kelvin temperature when the volume of the gas is changed to 1480 mL and the pressure is changed to 36.7 kPa? P1V1/T1 = P2V2/T2 99.0kPa(484 mL) = 36.7kPa(1480mL) 293K T2 47916T2 = 15914588 47916 47916 T2 = 332 K 9) Why does the volume of a gas decrease when the temperature decreases? a) The particles don’t move around as fast, so they need less space. 10) A balloon contains 5.5 L of air at 313 K and 101.3 kPa. After an hour, the air inside the balloon cools to 277 K. What is the final volume of the air in the balloon? Pressure is constant so use an equation without P V1/T1 = V2/T2 5.5 L = V2 313K 277 K 1523.5 = 313V2 313 313 4.9 L = V2 Potential Energy Diagrams 11) Draw an endothermic and exothermic potential energy diagram. Label reactants, products, change in enthalpy, energy of reactants, energy of products, activation energy and activated complex. Include a catalyst. How does a catalyst affect the reaction? A catalyst reduces/lowers the activation energy. 12) Given the energy diagram below, what information can be obtained from it? This reaction is endothermic. The reactants contain 80kJ of energy, the products contain 160 kJ. Therefore ΔH = 160-80 = 80kJ of energy. Ea without a catalyst = activated complex – reactants = 240-80 = 160 kJ Ea with a catalyst = activated complex with catalyst – reactants = 200 – 80 = 120kJ 13) Pair endothermic and exothermic reactions with the correct change in enthalpy : positive / negative. a) Endothermic- positive enthalpy; Exothermic- negative enthalpy Naming 14) What is the name of the compound N2O3? a) Dinitrogen Trioxide 15) What is the chemical formula for the compound tetraphosphorus decasulfide? a) P4S10 16) What is the name of the compound with the formula FeCl3? a) Iron (III) Chloride 17) What is the name for SnO2? a) Tin (IV) Oxide 1 2 3 4 5 mono di tri tetra penta 6 7 8 9 10 hexa hepta octa nona deca 18) What is the name of the compound with (NH4)2CO3? a) Ammonium Carbonate 19) Which is the formula for Calcium hydroxide? a) Ca(OH)2 20) What is the chemical formula for sulfuric acid? a) H2SO4 21) What is the formula for lead (II) chromate? a) PbCrO4 22) What is the chemical formula for hydrochloric acid? a) HCl Electrons 23) According to Niels Bohr's atomic model, what occurs when an atom absorbs radiated energy? a) The electron jumps to a higher energy level (excited state) 24) A hydrogen atom emits a photon of energy. Explain how this can happen. a) The electron jumped down to the ground state. 25) According to Bohr's model, what can be said of the amount of energy that an electron absorbs when it is excited compared to the amount of energy that it releases when it returns to ground state? a) The amount of energy is equal. 26) Using the Bohr Diagram on your reference sheet, what color is emitted by an electron that drops from n= 4 to n= 2? a) 486 nm corresponds to blue light. 27) Using the Bohr Diagram on your reference sheet, what energy level transition is indicated when the light emitted by a hydrogen atom has a wavelength of 103 nm? a) n=3 to n=1 28) Electron X can change to a higher energy level or a lower energy level. Explain what has to happen in either scenario. a) The electron absorbs energy to move to a higher energy level and releases energy to move to the lower energy level (ground state) 29) When the electron of a hydrogen atom moves into a higher energy orbit, what is the state of the atom? a) Excited state 30) What happens when a hydrogen atom changes from the excited state to the ground state? a) A photon is released. 31) When an electron moves from n= 3 to n=2, what wavelength of energy is emitted? a) 656 nm, red light 32) An electron moves from n= 4 to n= 1. What region of the EM spectrum is the wavelength located? a) 97 nm, ultraviolet Bonding 33) Describe metallic bonding (what is unique about the electrons). What are the properties of metallic bonds? a) Sea of mobile, delocalized electrons. Good conductors, high melting point, luster, ductility 34) Describe ionic bonding (what is unique about the electrons). What are the properties of ionic bonds? a) Electrons are transferred. Conducts in solution only. High melting/boiling points 35) Describe covalent bonding (what is unique about the electrons). What are the properties of covalent bonds? a) Electrons are shared. Low melting/boiling points, do not cocnduct, volatile. 36) What are the 5 major shapes of covalent molecules? How can you determine the shape? a) Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral. One bond: Linear Two bonds (1) with a lone pair of electrons- bent (2) no lone pairs- linear Three bonds (1) With a lone pair of electrons- triganal pyramidal (2) No lone pairs- trigonal planar Four bonds – Tetrahedral 37) What is the difference between polar and nonpolar molecules? a) Polar molecules- unequal sharing of electrons/have sides that look different b) Nonpolar molecules- equal sharing of electrons/ all sides look the same Electron Configuration 38) Write the electron configuration of nickel. 1s22s22p63s23p64s23d8 39) Write the noble gas configuration for Cu. [Ar]4s23d9 40) Write the orbital notation for N. __↑↓_ __↑↓_ __↑__ __↑_ 1s 2s 2p 2p __↑__ _____ _____ _____ _____ _____ _____ _____ _____ _____ 2p 3s 3p 3p 3p 4s 3d 3d 3d 3d 3d IRE 41) What is the definition of electronegativity? a) The tendency to attract electrons. 42) What is the trend in electronegativity across a period? Down a group? a) Across a period: electronegativity increases due to nuclear charge. Down a group: electronegativity decreases due to electron shielding. 43) What is the definition of ionization energy? a) The amount of energy needed to remove an electron. 44) What is the trend in ionization energy across a period? Down a group? a) Across a period: increases due to nuclear charge. Down a group: decreases due to electron shielding. 45) What is the definition of atomic radius? a) Measure of the size of the atom or half the distance between the nuclei of two identical atoms bonded together. 46) What is the trend in atomic radius across a period? Down a group? a) Across a period: decreases due to nuclear charge. Down a group: Increases due to electron shielding. Periodic Table 47) List the main families and their respective oxidation numbers/ionic charges. a) Alkali Metals (Group 1A) +1, Alkaline Metals (Group 2A) +2, Halogens (Group 7A) -1, Noble gases (Group 8A) No charges, Transition Metals (Group Bs) variable charges, Inner Transition Metals (Rows at bottom of periodic table) variable charges. 48) List at least 2 similarities for each family. a) Oxidation numbers/charges, valence electrons 49) What elements are metalloids? What are metalloids? a) Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, Astatine. Metalloids share properties with both metals and nonmetals. 50) Looking at the periodic table, list 4 atomic numbers that represent elements with similar chemical properties. Why did you choose those numbers? a) Any 4 numbers within the same family/group. Ex: 4, 20, 38, 56 Chemical Reactions 51) Predict the products for: Copper and HCl, Magnesium and HCl and Iron and HCl. What will you observe as a result of these reactions? Imagine that small pieces of the metals copper, magnesium and iron are placed in three test tubes labeled A, B, and C, respectively. If enough aqueous HCl is added to each tube to completely submerge the metal pieces, predict the products that will form in each test tube. a) No reaction for copper, you will observe bubbles in the other 2 reactions- products are MgCl2 and H2 and FeCl3 and H2 52) Predict the products resulting from the decomposition of sodium chlorate. For decomposition of a compound with a polyatomic ion, use your reference guidelines. 2NaClO3 2NaCl + 3O2 53) Write a balanced equation when Lithium and oxygen react in a synthesis equation. 4Li(s) + O2(g) 2Li2O(s) 54) Write the reaction that occurs between Fe(NO3)3 and KOH. a) Balance the reaction Fe(NO3)3(aq) + 3KOH(aq) Fe(OH)3(s) + 3KNO3(aq) b) Write the complete ionic equation. Fe+3(aq) + 3NO3-1(aq) + 3K+1(aq) + 3OH-1(aq) Fe(OH)3(s) + 3K+(aq) + NO3-1(aq) c) Write the net ionic equation. Fe+3(aq) + 3OH-1(aq) Fe(OH)3(s) Stoichiometry 55) Fluorine gas can react with ammonia gas to produce dinitrogen tetrafluoride gas and hydrogen fluoride gas: 5 F2 (g) + 2 NH3 (g) N2F4 (g) + 6 HF (g). What mass of hydrogen fluoride gas is produced from 335 g of ammonia gas assuming that there is an ample amount of fluorine gas present for the reaction to be completed? 1 𝑚𝑜𝑙 𝑁𝐻 6 𝑚𝑜𝑙 𝐻𝐹 20.01 𝑔 𝐻𝐹 335 g NH3 x 17.04 𝑔 𝑁𝐻3 x2 𝑚𝑜𝑙 𝑁𝐻 x 1 𝑚𝑜𝑙 𝐻𝐹 = 1180 g HF 3 3 56) How many moles of nitrogen would be needed to completely react with 5 moles of hydrogen according to the balanced equation given? N2 + 3H2 2NH3 1 𝑚𝑜𝑙 𝑁 5 mol H2 x 3 𝑚𝑜𝑙 𝐻2 = 1.67 mol N2 with sig figs 2 moles N2 2 57) If 22 grams of NH3 are used in this reaction, how many Liters of nitrogen (N2) will be produced? (Use the equation from the previous question) 1 𝑚𝑜𝑙 𝑁𝐻 1 𝑚𝑜𝑙 𝑁 22.4 𝐿 𝑁 22 g NH3 x 17.04 𝑔 𝑁𝐻3 x 2 𝑚𝑜𝑙 𝑁𝐻2 x 1 𝑚𝑜𝑙 𝑁2 = 15 L N2 3 3 2 58) What is the percent by mass of oxygen in Al2O3 ? Al: 2 x 26.98 = 53.96 Oxygen = 48.00 x 100 = 47.08% Oxygen O: 3 x 16 =48.00 101.96 Total = 101.96 g/mol 59) The molar mass of a compound is 92.0 g/mol. It contains 14.7 g N and 33.6 g O. What is its molecular formula? 1 𝑚𝑜𝑙 𝑁 1.05 𝑚𝑜𝑙𝑒 14.7 g N x = =1 14.01 𝑔 𝑁 1.05 𝑚𝑜𝑙𝑒 1 𝑚𝑜𝑙 𝑂 2.10 𝑚𝑜𝑙𝑒 33.6 g O x16.00 𝑔 𝑂 x 1.05 𝑚𝑜𝑙𝑒 = 2 Empirical Formula = NO2 = 1(14.01) + 2(16.00) = 46.01 g/mol. Molecular Mass = 92.0 = 2 Empirical Mass 46.01 Molecular Formula = 2(empirical) = N2O4 60) What is the empirical formula of a compound that contains 75% C and 25% H by mass? 1 𝑚𝑜𝑙𝐶 6.24 𝑚𝑜𝑙 1 𝑚𝑜𝑙 𝐻 24.75 𝑚𝑜𝑙 75 g C x12.01 𝑔 𝐶 = 6.24 𝑚𝑜𝑙 = 1 25 g H x1.01 𝑔 𝐻 = 6.24 𝑚𝑜𝑙 = 4 Empirical Formula = CH4 61) If a sample of magnesium has a mass of 45.0 g, how many atoms of magnesium does the sample contain? 1 𝑚𝑜𝑙 𝑀𝑔 45.0 g Mg x 24.31 𝑔 𝑀𝑔 x 6.022𝑥1023 𝑎𝑡𝑜𝑚𝑠 1 𝑚𝑜𝑙𝑒 𝑀𝑔 =1.11x1024 atoms 62) What is the percent by mass of oxygen in Beryllium oxide, BeO? 16.00 Be x 1 = 9.01 Oxygen = 25.01 x 100 = 63.97% O x 1 = 16.00 25.01 g/mol 63) What would be the empirical formula of a compound that is 25.5% carbon, 6.40% hydrogen, and 68.1% oxygen? 1 𝑚𝑜𝑙 𝐶 2.12 𝑚𝑜𝑙 25.5 g C x 12.01 𝑔 𝐶 = 2.12 𝑚𝑜𝑙 = 1 1 𝑚𝑜𝑙 𝐻 6.34 𝑚𝑜𝑙 6.40 g H x 1.01 𝑔 𝐻 = 2.12 𝑚𝑜𝑙 = 3 1 𝑚𝑜𝑙 𝑂 4.27 𝑚𝑜𝑙 68.1 g O x 16.00 𝑔 𝑂 = 2.12 𝑚𝑜𝑙 = 2 Empirical Formula = CH3O2 Solutions, Acids and Bases 64) When 0.50 liter of a 7 M solution is diluted to 1.0 liters, what will the new Molarity of solution be? M1V1 = M2V2 7 x 0.50 = M2 x 1.0 3.5 M = M2 65) What is the total number of grams of KOH needed to make 1.0 liter of a 0.25 M solution? a) Solve for Moles b) Convert Moles to Grams 56.11 𝑔 M = mol/L 0.25 moles x = 14 grams KOH 1 𝑚𝑜𝑙𝑒 0.25 M = x 1.0 0.25 mol = x 66) The pH of an unknown solution is 8. Is it an acid or base? What is its pOH? a) Base. pOH is 6 67) What primarily determines the pH of a solution? a) Concentration of hydrodium/hydrogen ions 68) What is the pH of a solution that has an OH- ion concentration of 1× 10-3 mole per liter? Is it an acid or base? a) pH of 11, base 69) What is the H+ of an HCl solution if the pH is measured to be 4? a) 1 x 10-4 70) What is a characteristic of a strong acid? a) Good electrolyte 71) What is the difference between strong and weak acids? a) Strong acids ionize 100%. Weak acids partially ionize. 72) What is the Bronsted-Lowry definition of an acid? Base? How is water classified? a) Proton donor- acid. Proton acceptor- base. Water is amphoteric… can be either an acid or base, depending on what it is paired with. Use the following Solubility Graph to answer questions 73) Which compound is least soluble in water at 40.°C? most soluble? a) Least soluble- SO2 Most Soluble- KI 74) Under which conditions are gases most soluble in water? a) Low temperature and high pressure. 75) How do you read a solubility graph? The solubility of a saturated solution of a solute (typically per every 100 grams of water) is represented by the lines on the graph, which changes depending on temperature. 76) According to Reference Table G, a temperature change from 60°C to 90°C has the least effect on the solubility of a) SO2 77) How do you know if a solution is saturated, unsaturated, or supersaturated? a) Saturated- on the line, unsaturated- below the line, supersaturated- above the line. 78) What are colligative properties? What properties are changed, and how? a) Properties that changed as a result of the solute. Osmotic pressure, increased boiling point, decreased freezing point, decreased vapor pressure. b) The effect increases as the number of solute particles increases. For example the boiling point of 1.0 M NaCl is higher than 1.0 M C6H12O6, because NaCl dissociates into two ions, so the molarity of solute particles is actually 2.0 M. Glucose (C6H12O6) doesn’t ionize therefore the molarity of the solute particles is 1.0M and the colligative properties are less than that of salt.