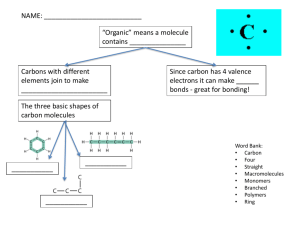
Organic Chemistry
advertisement

Chapter 3: pp. 37-58 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 10th Edition Sylvia S. Mader The Chemistry of Organic Molecules BIOLOGY © The McGraw Hill Companies, Inc./John Thoeming, photographer PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display 1 Outline Organic vs Inorganic Functional Groups and Isomers Macromolecules Carbohydrates Lipids Proteins Nucleic Acids 2 Organic Molecules Organic molecules contain carbon and hydrogen atoms bonded to other atoms Organic molecules are a diverse group Four types of organic molecules (biomolecules) exist in organisms: Carbohydrates Lipids Proteins Nucleic Acids 3 Organic versus Inorganic Molecules 4 Carbon Atom Carbon atoms: Contain a total of 6 electrons Only four electrons in the outer shell Very diverse as one atom can bond with up to four other atoms Often bonds with other carbon atoms to make hydrocarbons Can produce long carbon chains like octane Can produce ring forms like cyclohexane 5 Octane & Cyclohexane Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. octane cyclohexane 6 Functional Groups Functional groups are clusters of specific atoms bonded to the carbon skeleton with characteristic structure and functions Always react in the same manner, regardless of where attached Determine activity and polarity of large organic molecules Many functional groups, but only a few are of major biological importance Depending on its functional groups, an organic molecule may be both acidic and hydrophilic Nonpolar organic molecules are hydrophobic (cannot dissolve in water) unless they contain a polar functional group 7 Isomers Isomers - organic molecules that have: Identical molecular formulas, but Differing internal arrangement of atoms Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. glyceraldehyde H H H O C C C OH OH H dihydroxyacetone H H O H C C C OH H OH 8 Hexose Isomers 9 Macromolecules Carbohydrates, lipids, proteins, and nucleic acids are called macromolecules because of their large size. Usually consist of many repeating units Resulting molecule is a polymer (many parts) Repeating units are called monomers E.g. amino acids (monomer) are linked to form a protein (polymer) Some examples: Category Example Subunit(s) Lipids Fat Glycerol & fatty acids Carbohydrates Polysaccharide Monosaccharide Proteins Polypeptide Nucleic Acids DNA, RNA Amino acid Nucleotide 10 Common Foods Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. © The McGraw Hill Companies, Inc./John Thoeming, photographer 11 Dehydration and Hydrolysis Dehydration - Removal of water molecule Hydrolysis - Addition of water molecule Used to connect monomers together to make polymers Polymerization of glucose monomers to make starch Used to disassemble polymers into monomer parts Digestion of starch into glucose monomers Specific enzymes required for each reaction Accelerate reaction Are not used in the reaction Are not changed by the reaction 12 Dehydration Short polymer Unlinked monomer Dehydration reaction Longer polymer Hydrolysis Hydrolysis Dehydration and Hydrolysis 15 Synthesis and Degradation of Polymers Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. monomer OH H dehydration reaction monomer monomer H2O monomer a. Synthesis of a biomolecule monomer OH hydrolysis reaction monomer H monomer H2O monomer b. Degradation of a biomolecule 16 Carbohydrates Monosaccharides: Are a single sugar molecule such as glucose, ribose, deoxyribose Are with a backbone of 3 to 7 carbon atoms (most have 6 carbon). Disaccharides: Contain two monosaccharides joined by dehydration reaction Lactose is composed of galactose and glucose and is found in milk. Sucrose (table sugar) is composed of glucose and fructose Polysaccharides - Are polymers of monosaccharides Polysaccharides as Energy Storage Molecules Starch, Glycogen Polysaccharides as Structural Molecules Cellulose, Chitin 17 Popular Models for Representing Glucose Molecules Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CH2OH O H H CH2OH O H H C H C C OH H HO C C OH a. H OH H OH HO b. OH H OH C6H12O6 O c. O © Steve Bloom/Taxi/Getty d. 18 Synthesis and Degradation of Maltose, a Disaccharide Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CH2OH CH2OH O H H OH + CH2OH O dehydration reaction O O H2O maltose C12H22O11 water hydrolysis reaction HO glucose C6H12O6 monosaccharide CH2OH O glucose C6H12O6 monosaccharide disaccharide + water Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CH2OH CH2OH O glucose O O sucrose fructose CH2OH 19 Carbohydrates: Monosaccharides Single sugar molecules Quite soluble and sweet to taste Examples Glucose (blood), fructose (fruit) and galactose Hexoses - Six carbon atoms Isomers of C6H12O6 Ribose and deoxyribose (in nucleotides) Pentoses – Five carbon atoms C5H10O5 & C5H10O4 20 Carbohydrates: Disaccharides Contain two monosaccharides joined by dehydration reaction Soluble and sweet to taste Examples Lactose is composed of galactose and glucose and is found in milk Sucrose (table sugar) is composed of glucose and fructose Maltose is composed of two glucose molecules 21 Carbohydrates: Polysaccharides Polymers of monosaccharides Low solubility; not sweet to taste Polysaccharides as Energy Storage Molecules Starch found in plant Polymer of glucose Few side branches Used for short-term energy storage Amylose (unbranched) and amylopectin (branched) are the two forms of starch found in plants Glycogen is the storage form of glucose in animals. Highly branched polymer of glucose with many side branches Glycogen in liver and muscles 22 Carbohydrates: Polysaccharides Polysaccharides as Structural Molecules Cellulose is a polymer of glucose which forms microfibrils Primary constituent of plant cell walls Main component of wood and many natural fibers Indigestible by most animals Chitin is a polymer of glucose with an amino group attached to each glucose Very resistant to wear and digestion Primary constituent of arthropod exoskeletons (e.g. Crab) and cell walls of fungi 23 Complex Carbohydrates 24 Starch Structure and Function Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. starch granule a. Starch 250mm © Jeremy Burgess/SPL/Photo Researchers, Inc. 25 Glycogen Structure and Function Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. glycogen granule 150 nm b . Glycogen © Don W. Fawcett/Photo Researchers, Inc. 26 Cellulose Structure and Function Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. cellulose fiber microfibrils Plant cell wall cellulose fibers 5,000 m glucose molecules © Science Source/J.D. Litvay/Visuals Unlimited 27 Carbohydrates as Structural Materials Plants cell wall consist of cellulose Cell wall of fungi and shell of crab contain chitin Bacterial cell wall contain peptidoglycan Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. b. Shell contains chitin. a. Cell walls contain cellulose. c. Cell walls contain peptidoglycan. a: © Brand X Pictures/PunchStock; b: © Ingram Publishing/Alamy; c: © H. Pol/CNRI/SPL/Photo Researchers, Inc. 28 Lipids Lipids are varied in structure Insoluble in water Long chains of repeating CH2 units Renders molecule nonpolar Lack polar groups 29 Lipids Fat provides insulation and energy storage in animals Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. © Paul Nicklen/National Geographic/Getty 30 Types of Lipids 31 Types of Lipids: Triglycerides Fats and oils contain two molecular units: glycerol and fatty acids. Dehydration Synthesis of Triglyceride from Glycerol and Three Fatty Acids Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. H H C H C O OH OH H H H H H C C C C H C H H H H H H H H H H C C C C C C C C HO + HO C OH H glycerol a. Formation of a fat dehydration reaction O O H C H HO H H H H C C H H H H H H C kink 3 fatty acids O O H H H H C C C C C H H H H O H H H H H H C C C C C C C H H H H H H O H H H H + 3 HO 2 hydrolysis reaction H C H H O O C C C H kink fat molecule 3 water molecules 32 Types of Lipids: Triglycerides Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. corn corn oil H H H H H H H C C C C C C C C H H H H H H H H H H C C C H H H H C C C H H H H H C C C H H O C HO H unsaturated fatty acid with double bonds (yellow) unsaturated fat milk butter H H H H H H H H H H H H H H H H C C C C C C C C C C C C C C C C C H H H H H H H H H H H H H H H H O HO saturated fatty acid with no double bonds b. Types of fatty acids H saturated fat c. Types of fats 33 Types of Lipids: Triglycerides Triglycerides (Fats) Long-term energy storage Consist of a backbone of one glycerol molecule Glycerol is a water-soluble compound with three hydroxyl groups. Three fatty acids attached to each glycerol molecule Long hydrocarbon chain Saturated - no double bonds between carbons e.g. in fats (butter) Unsaturated - 1 or more than1 double bonds between carbons e.g. in oils Carboxylic acid at one end Carboxylic acid connects to –OH on glycerol in dehydration reaction 34 Animal and Plant Fats Most plant fats are unsaturated and most animal fats are saturated. Butter and lard are solids at room temperature. Too much saturated and trans fats can lead to atherosclerosis. Lipids containing deposits called plaques build up within the walls of blood vessels, reducing blood flow. Atherosclerosis Types of Lipids: Phospholipids Phospholipids Derived from triglycerides Glycerol backbone Two fatty acids attached instead of three Third fatty acid replaced by phosphate group The fatty acids are nonpolar and hydrophobic The phosphate group is polar and hydrophilic Molecules self arrange when placed in water Polar phosphate “heads” next to water Nonpolar fatty acid “tails” overlap and exclude water Spontaneously form double layer & a sphere 37 Types of Lipids: Phospholipids Phospholipids Form Membranes Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. glycerol O Polar Head –O R O P O 1CH 2 O 2CH O CH2 CH2 CH2 CH CH 2 2 CH2 CH 2 CH2 CH 3 fatty acids 3CH 2 O CH2 CH2 CH CH = 2 CH2 CH2 CH2 CH2 O Nonpolar Tails outside cell inside cell phosphate a. Phospholipid structure b. Plasma membrane of a cell 38 Types of Lipids: Steroids & Waxes Steroids Cholesterol, testosterone, estrogen Skeletons of four fused carbon rings Waxes Long-chain fatty acid bonded to a long-chain alcohol High melting point Waterproof Resistant to degradation 39 CONNECTION Anabolic • steroids pose health risks Anabolic steroids are natural and synthetic variants of the male hormone testosterone – Build up bone and muscle mass – Can cause serious health problems Anabolic Steroids Anabolic steroids are synthetic variants of the male hormone testosterone – which generally causes a build up in muscle mass in males during puberty and maintains masculine traits. Positive uses of anabolic steroids As a prescription drug, anabolic steroids are used to treat general anemia and diseases that destroy body muscle. Negative effects of Anabolic Steroids • • • • • Violent mood swings (“Roid Rage”) Deep Depression Alter cholesterol levels leading to high blood pressure and increasing risk of cardiovascular problems. Reduces the bodies natural output of male sex hormones – shrunken testicles, reduced sex drive, infertility, and breast enlargement in men. In women, use has been linked to menstrual cycle disruption and development of masculine characteristics. Waxes Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. a. b. a: © Das Fotoarchiv/Peter Arnold, Inc.; b: © Martha Cooper/Peter Arnold, Inc. 43 Proteins Functions Support proteins Keratin - makes up hair and nails Collagen - support many of the body’s structures e.g. tendons, skin Enzymes – Almost all enzymes are proteins Acts as organic catalysts to accelerate chemical reactions within cells Transport – Hemoglobin; membrane proteins Defense – Antibodies Hormones are regulatory proteins that influence the metabolism of cells e.g. insulin Motion – Muscle proteins, microtubules 44 Protein Subunits: The Amino Acids Proteins are polymers of amino acids Each amino acid has a central carbon atom (the alpha carbon) to which are attached a hydrogen atom, an amino group –NH2, A carboxylic acid group –COOH, and one of 20 different types of –R (remainder) groups There are 20 different amino acids that make up proteins Amino acids differ according to their particular R group 45 Protein Structure 46 Structural Formulas for the 20 Amino Acids Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Sample Amino Acids with Nonpolar (Hydrophobic) R Groups H H H3N+ O C H3N+ C (CH2)2 O– H O C C CH H3C H3N+ O H O H3N+ C C O– CH2 CH methionine (Met) phenylalanine (Phe) H H2N+ C H2C CH2 CH3 CH3 CH3 valine (Val) H O– CH2 S CH3 H C C C O– CH2 leucine (Leu) proline (Pro) Sample Amino Acids with Polar (Hydrophilic) R Groups H H3N+ H O H3N+ C C O– CH2 C H O H3N+ C O– CH2 H O C H3N+ C O– CH2 H3N+ NH2 O C C CH2 H O O– H3N+ O– CH OH O asparagine (Asn) O glutamine (Gln) OH tyrosine (Tyr) C C C NH2 O– C serine (Ser) H C (CH2)2 OH SH cysteine (Cys) O C CH3 threonine (Thr) Sample Amino Acids with Ionized R Groups H H H3N+ C O H3N+ C CH2 C O– H O C H O– H3N+ O C CH2 CH2 CH2 C COO– N+H3 CH2 glutamic acid (Glu) lysine (Lys) –O C O O– O O– H3N+ O C C CH2 NH C aspartic acid (Asp) H C (CH2)3 C CH2 H3N+ N+H2 NH2 arginine (Arg) O– NH N+H histidine (His) 47 Proteins: The Polypeptide Backbone A peptide bond is a covalent bond between two amino acids (AA) COOH of one AA covalently bonds to the NH2 of the next AA Two AAs bonded together – Dipeptide Three AAs bonded together – Tripeptide Many AAs bonded together – Polypeptide Characteristics of a protein determined by composition and sequence of AA’s A protein may contain more than one polypeptide chain Virtually unlimited number of proteins 48 Synthesis and Degradation of a Peptide Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. amino group peptide bond acidic group dehydration reaction hydrolysis reaction amino acid amino acid dipeptide water Protein: Levels of Structure Protein shape (3-D structure) determines the function of the protein in the organism Proteins can have up to four levels of structure Primary: Secondary: The way the amino acid chain coils or folds Describing the way a knot is tied Tertiary: Literally, the sequence of amino acids A string of beads (up to 20 different colors) Overall three-dimensional shape of a polypeptide Describing what a knot looks like from the outside Quaternary: Consists of more than one polypeptide Like several completed knots glued together 50 Levels of Protein Organization Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. H3N+ Primary Structure amino acid This level of structure is determined by the sequence of amino acids that join to form a polypeptide. peptide bond COO– hydrogen bond hydrogen bond Secondary Structure Hydrogen bonding between amino acids causes the polypeptide to form an alpha helix or a pleated sheet. (alpha) helix (beta) sheet = pleated sheet Tertiary Structure Due in part to covalent bonding between R groups the polypeptide folds and twists giving it a characteristic globular shape. disulfide bond Quaternary Structure This level of structure occurs when two or more polypeptides join to form a single protein. 51 Animation Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. 52 Examples of Fibrous Proteins Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. a. b. c. a: © Gregory Pace/Corbis; b: © Ronald Siemoneit/Corbis Sygma; c: © Kjell Sandved/Visuals Unlimited 53 Protein-folding Diseases Assembly of AA’s into protein extremely complex Process overseen by “chaperone” molecules Inhibit incorrect interactions between R groups as polypeptide grows Defects in these chaperones can corrupt the tertiary structure of proteins Mad cow disease could be due to mis-folded proteins 54 Nucleic Acids Polymers of nucleotides Very specific cell functions DNA (deoxyribonucleic acid) Double-stranded helical spiral (twisted ladder) Serves as genetic information center In chromosomes RNA (ribonucleic acid) Part single-stranded, part double-stranded Serves primarily in assembly of proteins In nucleus and cytoplasm of cell 55 The Nucleotides of Nucleic Acids Three components: A phosphate group, A pentose sugar (ribose or deoxyribose), and A nitrogenous base (4 kinds in DNA, 3 kinds in RNA, 3 common to both Nucleotide subunits connected end-to-end to make nucleic acid Sugar of one connected to the phosphate of the next Sugar-phosphate backbone 56 Nucleotides Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. nitrogencontaining base phosphate pentose sugar a. Nucleotide structure deoxyribose (in DNA) ribose (in RNA) b. Deoxyribose versus ribose Purines Pyrimidines cytosine thymine in DNA c. Pyrimidines versus purines uracil in RNA adenine guanine 57 DNA Structure Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. A G T T C C A G C Cytosine S Sugar G Guanine A Adenine P Phosphate T Thymine a. Space-filling model b. Double helix © Photodisk Red/Getty Images 58 RNA Structure Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. P S Nitrogen-containing bases P S Backbone P C Cytosine S Ribose G Guanine A Adenine S P Phosphate U Uracil P S 59 Complementary Base Pairing Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. sugar N guanine (G) sugar cytosine (C) sugar sugar adenine (A) thymine (T) c. Complementary base pairing 60 Comparison of DNA & RNA 61 Other Nucleic Acids ATP (adenosine triphosphate) is composed of adenine, ribose, and three phosphates In cells, one phosphate bond is hydrolyzed – Yields: The molecule ADP (adenosine diphosphate) An inorganic phosphate molecule pi Energy Other energy sources used to put ADP and pi back together again 62 ATP Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. a. adenosine triphosphate c. H2O P adenosine b. P P triphosphate ATP P adenosine P diphosphate + P + energy phosphate ADP c: © Jennifer Loomis/Animals Animals/Earth Scenes 63 Review Organic vs Inorganic Functional Groups and Isomers Macromolecules Carbohydrates Lipids Proteins Nucleic Acids 64 Chapter 3: pp. 37-58 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 10th Edition Sylvia S. Mader The Chemistry of Organic Molecules BIOLOGY © The McGraw Hill Companies, Inc./John Thoeming, photographer PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display 65