From Particles to Solutions
advertisement

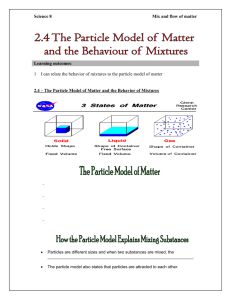
From Particles to Solutions Chemistry 01 Learning Goals!! • I will be able to explain the particle theory of matter. • I will be able to classify matter using the terms: pure substance, element, compound, mechanical mixture and solution What is CHEMISTRY!? • The study of matter and the changes it undergoes Matter … • Anything that has mass and takes up space. The Particle Theory of Matter • • • • • All matter is made up of different kinds of particles Different substances are made up of different types of particles Particles are in constant random motion Particles move faster when heated, and slower when cooled Particles attract each other Lists • Create a list of 15-20 items you’ve used, eaten, seen, etc. in the last day or two. • Save this list for later. Pure Substances • Made up of only one type of particles • Any element from the Periodic Table • Any compound (like distilled water, carbon dioxide) Mixtures • Made up 2 or more types of particles • Mechanical mixture – you are able to see the different particles • pizza, oil and vinegar, cereal • Solution – a mixture where you can’t see the different particles and you can see through it • apple juice, air in this room • Alloy – a solid solution of two or more metals. • i.e. Bronze -> Copper + Tin Classification of Matter MATTER PURE SUBSTANCES Can combine to form MIXTURES ELEMENTS COMPOUNDS SOLUTIONS i.e: H, O, C i.e: H2O, NaCl i.e: H2O + salt MECHNICAL MIXTURES i.e: H2O + raisins Learning Goals Revisited • I will be able to explain the particle theory of matter. • I will be able to classify matter using the terms: pure substance, element, compound, mechanical mixture and solution Task • Go back to your list. Try to determine what type of matter each item is. i.e. if you had pizza it would be a mechanical mixture. • Share list with your partner • Complete poem Qs • Textbook p178 #2-6, 8.