Ch.2 PS Notes (Part 1)
advertisement

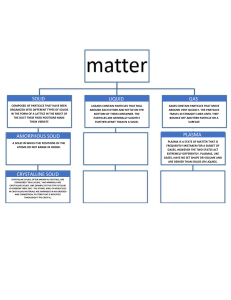
Science Notes Ch. 2: Solids, Liquids, and Gases (part 1) I. Matter is something that has mass and takes up space (volume). Matter makes up less than 5% of the universe. The rest consists of Dark Matter and Dark Energy. A. Matter exists in 4 states: i. Solid ii. Liquid iii. Gas iv. Plasma Examples of the 4 states of matter An ice cube is an example of a solid Water is an example of a liquid A cloud is an example of matter in the gas state The Earth atmosphere is another example of matter in the gas state Lightning is an example of matter in the plasma state The Sun is another example of matter in the plasma state Plasma is the most abundant form of matter in the Universe, because most stars are in a plasma state. II. Phases of Matter A. A Solid is a phase of matter that has a fixed shape and fixed volume. i. Solid atoms are fixed and closely packed causing solids to have a fixed shape and volume. ii. Types of solids 1. Crystalline solids – are solids that are made up of crystals. a. These crystals are made up of repeating, shape patterns. Example of the molecular structure of a “crystalline” solid. Notice how the same basic shape is repeated over and over. 2. Amorphous solids – are solids where the particles are not arranged in a pattern. Crystalline vs. Amorphous solid: notice how the amorphous solid does not have the same shape repeating over and over B. A Liquid is a phase of matter that has a definite (fixed) volume but no shape of its own. i. Liquid atoms are packed closely together but are free to move around each other while still touching. 1. Because its particles are free to move, a liquid has no definite or fixed shape. However, it does have a definite volume. 2. These free moving particles allow a liquid to flow. That’s why liquids are also called fluids. a. A Fluid – is a substance that flows. 3. Properties of Liquids a. Surface tension – is an inward force, or pull, among molecules in a liquid that brings the molecules on the surface closer together. i. Due to surface tension, the surface of water can act like a sort of skin. 1. Example: Beaker with water and Sewing needle over the surface of water. The atoms in H20 have a fixed volume (space they take up) but no fixed shape since they mold to their container and are always moving around each other.