Chapter 4: Calculations Used in Analytical Chemistry
advertisement

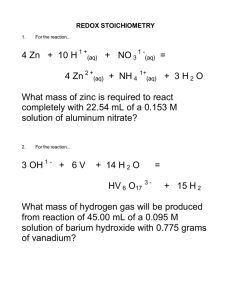
Chapter 4: Calculations Used in Analytical Chemistry CHE 321: Quantitative Chemical Analysis Dr. Jerome Williams, Ph.D. Saint Leo University Overview • • • • Units & SI System Moles & Millimoles Solutions: Expressing Concentration Stoichiometry Units & SI System • Units convey information and serve as guide to solving problems – Fundamental Units – Derived Units Table 4-1 p63 Units & SI System • Measurements based on SI system, which is metric system – Metric Prefixes – Performing Unit Conversions (see Tutorials) Table 4-2 p63 Moles & Millimoles • Mass versus Weight • Moles are the SI unit for amount of a substance. • One mole of anything contains Avogadro’s number of particles (chemical formula) p64 Moles & Millimoles • Molar Mass is mass in grams of one mole of a substance. • Calculate molar mass by summing atomic masses of all elements in formula. • Often more convenient to use unit millimole (Note: 1 mmole is equivalent to 10-3 mole) p65 Moles & Millimoles • Review Examples 4.1 & 4.2 • Complete Tutorials Solutions: Expressing Concentration • Review definitions of analytical molarity, and equilibrium molarity – Equilibrium concentrations expressed as molarity – Analytical concentrations use formal concentration • Review Examples 4.4 – 4.6 • Review Tutorials Solutions: Expressing Concentration • Percent Concentration - review definitions & formulas for the following – – – – – Mass (Weight)% Volume % Weight/volume % Parts per Million (ppm) Parts per Billion (ppb) • Go over example problems & complete tutorials Solutions: Expressing Concentration • Review definitions of p-Functions pX = - log [ X ] Solutions: Expressing Concentration • Review definitions of density and specific gravity • Density = mass / volume • M1 V1 = M2 V2 Figure 4-1 p74 Table 4-3 p74 Stoichiometry • Quantitative relationship among reactants & products in a balanced chemical reaction. • Review Empirical vs. Molecular Formulas • Review Structural Formulas Figure 4-2 p76 Suggested Problems • HW Set 2: 4.1, 4.4, 4.7-4.15 (odd) • HW Set 3: 4.17, 4.20, 4.23, 4.31, 4.35