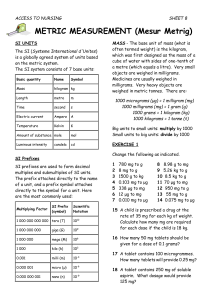
4 Zn + 10 H (aq) + NO3 4 Zn (aq) + 3 H2 O What mass of zinc is
advertisement

REDOX STOICHIOMETRY 1. For the reaction... 4 Zn + 10 H 1 +(aq) + NO 3 1 -(aq) = 4 Zn 2 +(aq) + NH 4 1+(aq) + 3 H 2 O What mass of zinc is required to react completely with 22.54 mL of a 0.153 M solution of aluminum nitrate? 2. For the reaction... 3 OH 1 - + 6 V + 14 H 2 O HV 6 O17 3 - = + 15 H 2 What mass of hydrogen gas will be produced from reaction of 45.00 mL of a 0.095 M solution of barium hydroxide with 0.775 grams of vanadium? 1. Nitrate anion is a reactant in this reaction. However, nitrate anion cannot exist as an independent entity, some counterbalancing cation must always be associated with the anion. In this case, a solution of aluminum nitrate furnishes the nitrate anion to the reaction, and the concentration of the solution is expressed in terms of Al(NO 3 ) 3 . So... moles zinc = moles aluminum nitrate [ C.F.'s] moles of ZINC (a solid substance) = moles aluminum nitrate (a solution) then: given mass ? grams = std . mass 65.4 g / mole = ( Molarity )(Vol. Liters) (? grams / 65.4) = ( 0.153 )(0.02254 L) [ C.F.'s] 3 mole NO31− 4 mole Zn [ C.F.'s] = 1− 1 mole Al ( NO3) 3 1 mole NO3 The FIRST C.F. is based on the chemical formula of aluminum nitrate, and relates ONE mole of compound aluminum nitrate, to THREE moles of nitrate anions. The SECOND C.F. is based on coefficients in the balanced chemical equation, and relates FOUR moles of zinc to ONE mole of nitrate anion. ? grams = 2.7065 grams zinc 2. Recognize this is a limiting reagent type of a problem, because amounts of both reactants are specified. The barium hydroxide solution furnishes hydroxide anions to the reaction. Since the molarity and volume of the solution are both given, and also because the formula of barium hydroxide is known, then the amount (i.e., moles) of hydroxide anion available to the reaction is also given. Similarly, because the mass of solid vanadium is given, and its standard mass is known, then the amount (i.e., moles) of vanadium is also given. Solution to this problem will be illustrated using the B4, REACT, and AFTR grid. Let's use millimole units, and calculate millimole quantities of hydroxide and vanadium: millimoles = ( Molarity ) ( Vol. in mL ) millimoles = ( mass in mg / Std.mass ) ? millimoles OH − = millimoles Ba(OH) 2 [ C.F. ] 2 mole OH − = (0.095 M)(45.00) = 8.55 mmoles OH − 1 mole Ba ( OH ) 2 ? millimoles V = 775 milligrams V 1 millimole 50.94 milligrams = 15.21 mmoles V set-up the reaction grid, and enter these starting amounts in the B4 row... REACTANTS, will be lost B4 REACT, in ratio of ( -3; -6; +15 ) AFTR PRODUCTS 15 H 2 3 OH − 6 V 8.55 15.21 none -7.61 -15.21 +38.025 0.94 none 38.025 Suppose millimoles hydroxide was picked as a starting point. How much vanadium would be needed to completely react with 8.55 millimoles of hydroxide anion? Let's figure it out... ? millimoles V = 8.55 millimoles OH − [ C.F. ] The relationship between V and OH − is given by coefficients in the balanced chemical redox equation; 6 V for every 3 OH −. So the C.F. is as shown... ? millimoles V = 8.55 millimoles OH − 6 millimoles V 3 millimoles OH − = 17.1 Thus 17.1 millimoles V is needed to react with all of the hydroxide anion present. However, only 15.21 millimoles V is actually present, so hydroxide anion must be the reagent present in an excess amount - or vanadium is the limiting reagent. Conclude that all vanadium will be used up in this reaction. The amount of hydroxide actually needed for this reaction will then be half the amount of vanadium reacted, because their coefficients are in the ratio 3 OH − for every 6 V. ( 3 / 6 ) of 15.21 = 7.61 millimoles OH − The amount of hydrogen formed will be more than the amount of vanadium according to the ratio 15 to 6, because their coefficients are in the ratio 15 H 2 for every 6 V. ( 15 / 6 ) of 15.21 = 38.025 millimoles H 2 Be certain that ALL quantities present in the REACT ROW are in the same proportion as coefficients in the balanced chemical equation. In this instance the proportions (via coefficients) are -3 OH, to -6 V, to +15 H 2 , are in the same proportions as (via millimole amounts) -7.61 millimoles OH −, to -15.21 millimoles V, to +38.025 millimoles H 2 . Finallly, what is the mass of 38.025 millimoles of hydrogen? millimoles = mass in milligrams standard mass 38.025 millimoles = ? milligrams / 2 ? milligrams H 2 = 76.05 or, ? grams H 2 = 0.076