Chemical Reactions & Balancing Equations
advertisement
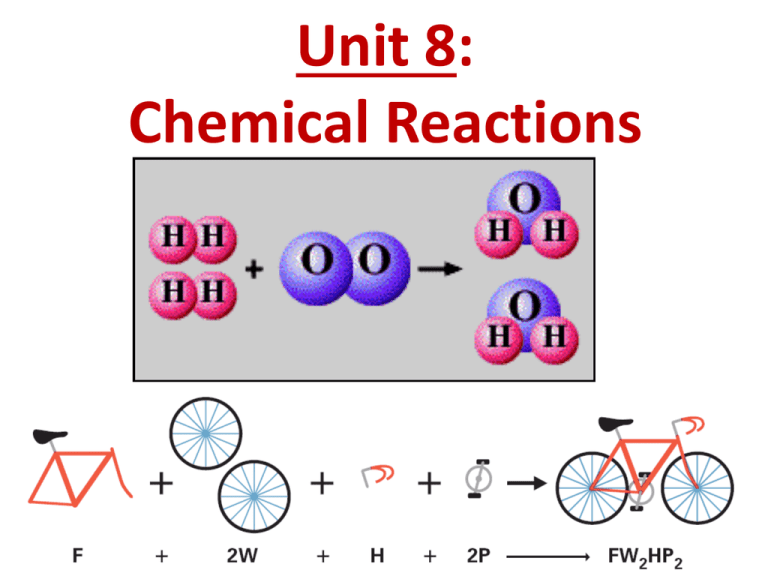
Unit 8: Chemical Reactions Changes of Matter • Physical Changes: Demo do not change the composition of a substance. • melting, vaporizing, dissolving, size, etc. • Chemical Changes: A B result in new substances. • burning, rusting, decomposition, etc. AB 4 Clues of Chemical Change Demo: Zn + H+ Zn2+ + 1) gas production Pb2+ + I– 2) color change Mg + O2 3) energy transfer (temp change, light) 4) precipitate formation (solid forms from solution) • Chemical Change: new & different substances formed. Chemical Reaction • Chemical Equation: A+B yields AB REACTANTS PRODUCTS present at the start of the rxn produced in the rxn at the end Law of Conservation of Mass • In a chemical reaction, matter is neither created nor destroyed. • Atoms are rearranged. 4H 4H 2O 2O Law of Conservation of Mass mass of reactants = mass of products ? Chemical Equations Balancing Equations WHY? mass is conserved (same before & after) N2H4 + O2 N2 + H2O N2H4 + O2 N2 + 2 H2O Coefficient - # of units of each substance Chemical Equations H2 + O2 H2O 3 Balancing Steps 1. Count atoms on each side. 2. Add Coefficients to make # of atoms equal. coefficient subscript = # atoms How many N’s in: 2 Fe(NO3)2 3. Check atom balance!!! Chemical Equations Aluminum and copper(II) chloride form copper and aluminum chloride. 2 Al + 3 CuCl2 3 Cu + 2 AlCl3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6 Quick Quiz! 1. Which of the following is a chemical reaction? A. melting of lead B. dissolving sugar in water C. rusting of iron (new substance) D. crushing of stone Quick Quiz. 2. Which of the following is NOT a possible clue that a chemical change is taking place? A. B. C. D. a change of state a change in color production of a gas formation of a precipitate Quick Quiz. 3. Why do we have to balance equations? A. B. C. D. it’s so much fun! it’s the law of the west it’s the law of consternation of moose it’s the law of conservation of mass Quick Quiz. 4. During any chemical change, the mass of the final products is… A. always equal to the mass of the starting reactants. B. always greater than the mass of the starting reactants. C. always less than the mass of the starting reactants. D. sometimes different than the mass of the starting reactants. Quick Quiz. 5. What is the coefficient of HCl when the following reaction is balanced? 2__Al Al ++ 6__HCl HCl → __AlCl 2 AlCl33 ++ __H 3 H22 ___Al___ 1 1 ___H___ 1 2 ___Cl___ 3 1 A. 1 B. 2 C. 3 D. 6