Chemical Reaction Types & Atom Compound
advertisement

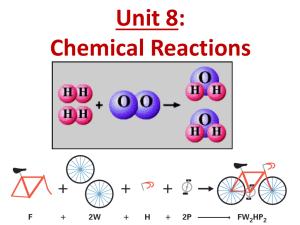
Grab binders, handouts & returned work & begin the Do Now 4/17 Do Now 1. Set you Periodic Tables at the corner of your desk for it to be checked by Ms. Herndon 2. Turn in your completed EXIT TICKET 2 & HW 1 to the class bucket. 3. Take 5 minutes to prepare for today’s quiz on bonding & chemical vs. physical reactions & properties Quiz 1 • 45 min to complete (we will track immediately after) • DO NOT write on the quiz, write answers on separate sheet of paper • When finished turn in the quiz upfront, grab a colored pencil & calculator, and sit silently. – Things to Do: • HW 2 • Lab 1: Copper Cycle Lab Report • Other course work Reaction Equation Recap: Phases 2H2O2(aq) 2H2O(l) + O2(g) Reactants Products - 1 reactant, 2 products - Chemical rxn NOT physical rxn What types of chemical reactions are there? Determining Types of Chemical Reactions • Arrange the slips into groups • HINT: There are 5 groups with 3 slips in each • Questions to consider: – What are your reactants and products? – How many reactants are there? – How many products are there? – Consider how the different reactions differ and how they are similar Decomposition (8, 9, 14) • Description: a compound breaks down to simpler products • General Equation: AB A + B • Example: 2NaCl 2Na + Cl2 • Question: More products than reactants? Synthesis (6, 11, 13) • Description: 2 or more simpler reactants join to form a compound • General Equation: A + B AB • Example: 2Na + Cl2 2NaCl Question: More reactants than products? Single Exchange (3, 7, 15) • Description: a single atom replaces another atom in a compound • General Equation: A + BC AC + B • Example: 2Na + CuCl 2NaCl + Cu Question: Do we see a single element on both sides? Double Exchange (2, 4, 12) • Description: atoms in compounds switch partners and end with 2 new compounds • General Equation: AB + CD AD + BC • Example: NaCl + LiBr NaBr + LiCl Question: 2 compounds of reactants and as products? Combustion (1, 5, 10) • Description: a CH compound burns in O2 to form CO2 and H2O (water) • Example: C2H4 + 3O2 2CO2 + 2H2O Question: are CO2 and H2O products? Counting Atoms in Compounds Step 1: Identify your elements in the compound Step 2: Distribute your subscripts Step 3: Distribute your coefficients across all elements a. 4PO4 b. 6C3H8O2 c. (CH4) 2N2 Counting Atoms Guided Practice How many atoms of each element are in the following molecules? 1. 2. 3. 4. 5. H2SO4 CaOH2 NaCl (NH3)3PO4 3H2O Answers 1. 2 hydrogen, 1 sulfur, 4 oxygen 2. 1 calcium, 1 oxygen, 2 hydrogen 3. 1 sodium, 1 chlorine 4. 3 nitrogen, 9 hydrogen, 1 phosphorus, 4 oxygen 5. 6 hydrogen, 3 oxygen