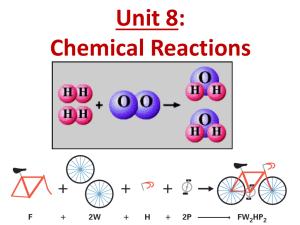
Goal(s) - Mr. P's AP Science Site
advertisement
Andrew D. Nicole R. Karla R. Breanna H. Ulises G. Samantha H. Nancy C. Isaias G. Liliana R. Horacio S. Jocelyn Q. Front of the Classroom Jackie C. Sarah R. Valerie S. Maddie R. Nacho H. Julia P. Nellie S. Gwendolyn F. Tyler R. Christopher G. Nelson O. Jasmine J. Back of the Classroom Dulce C. Gloria G. Brandon P. Autumn H. George S. Classroom Door 1) Bree, Dulce, Brandon, George 2) Maddie, Nancy, Horacio, Nacho 3) Andrew, Tyler, Jasmine, Nellie 4) Nicole, Valerie, Liliana, Samantha 5) Gwendolyn, Sarah, Julia, Karla 6) Ulises, Isaias, Jocelyn, Jackie 7) Autumn, Gloria, Chris, Nelson Back of the Classroom Abby S. Joshua G. Jacqueline A. Janice L. Marc C. Jalisa C. Isamar M. Yanni G. Brianne M. Mia C. Mariela P. Jeremy S. Monse M. Isaac S. Ashley G. Audrey O. Esmeralda C. Mileny A. Isabella G. Front of the Classroom AK Lesly R. Quinton K. Classroom Door 1) Quinton, Isaac, Mileny, Mia 2) Yanni, Audrey, Jeremy, AK 3) Joshua, Marc, Abby, Janice 4) Isabella, Mariela, Ashley, Jacqueline 5) Brianne, Isamar, Jalisa 6) Monse, Esmeralda, Lesly Back of the Classroom Zaria H. Deyanis B. Yezenia S. Vanesa G. Michael P. Uriel R. Josue C. Kevin R. Israel O. Andrea G. Yashley P. Isaiah C. Jasmin D. Eder A. Julisa T. Front of the Classroom Bryndan G. Marisol R. Sarah N. Rut M. Vincent R. Angelica M. Emily V. Classroom Door 1) Angelica, Emily, Bryndan, Vincent 2) Uriel, Julisa, Eder, Jasmin 3) Deyanis, Zaria, Vanesa, Yezenia 4) Isaiah, Israel, Andrea, Yashley 5) Marisol, Rut, Sarah 6) Kevin, Michael, Josue Back of the Classroom Group 2 Group 3 Group 7 Group 5 (for period B1) Group 1 Group 4 Group 6 Front of the Classroom Classroom Door 10/13/2015 1) How was your weekend? 2) Write the formula for the following: (a) Tricarbon octahydride (b) Barium nitride (c) Hydrophosphoric acid (d) Phosphoric acid (e) Iron (III) hydroxide -five volunteers for board *For next year, when ya’ll be in college Goal(s): Write chemical reactions Molarity calculations and demonstrating proper lab technique Agenda: 1) Do Now (show me page 116 & 162) 2) Writing Reaction Notes 3) Grade HW 4) HW Check 5) Continue Molarity challenge Need safety goggles for both 5 & 6 6) Ionic Compounds Lab (continue on Thursday) HW: Pg. 166-173; Pg 164: 4bd, 5c Pg 152 5) a. 0.0017 M C8H10N4O2 6) 3.6 g C6H12O6 Pg. 143 2) 39.7% is water! 6) BaCO3 b. 0.667 M C6H12O6 5 – a and b are worth one point each. Also note: molar = mol/L 7) Mass out 11 g of NaNO3 and add water up to 250 mL Water’s mass adds up to 54, and total mass is 136. 1 point for each box! 9) N2H4 Total the number wrong on page 143. 4) Total points: 11 Amount Wrong Percentage 0 to 2 100 3 95 4 90 HW 2.3 5 85 Count up the amount of marks they have, 6 80 and use the table to the right for a score Blank spaces for assignments page count for a mark! Write score on top of pg. 143 I sometimes with I was a bird 75 7 70 8 65 9 60 10 50 40 11 Uh so you didn’t do it 30 0 Check your answers Pg. 157: 2) CaO (s) + H2O (l) → Ca(OH)2(s) 3) Carbonic Acid decomposes to carbon dioxide gas and water Pg. 159: b, d, e – are correct! Pg. 162: 2) 2(NH4)3P (aq) + 3 Pb(BrO3)2 (aq) → Pb3P2 (s)+ 6 NH4BrO3 (aq) 3) 3 Ca (s) + Ni2(SO4)3 (aq) → 3 CaSO4 (aq) + 2 Ni (s) Lab Safety! Safety Glasses! 1. Show Calculations on what you will be do to make your solution. Get it checked 2. Make solution Clean glassware and equipment! Make sure the items are clean! Do not contaminate the solutions or the stock bottles of chemical! Can’t get the solid to all dissolve? Learn how to use stirring plate up front. 3. Make sure you clean all glassware at the end and return items to where you found them! Make sure your lab area is clean. 4. First three to finish (1st hour): Pour the stock solution into the small bottles. Label them! -Write your substance that you dissolved in water. -Your substance breaks apart into it’s ions when in water!! -If your substance had a hydrate (don’t put the hydrate in the reactant side) -If you need to balance it, balance it! 10/13/2015 1) Balance the following reaction: Mg + O2 MgO 2) Label the type of reaction: 2 K + CuF2 2 KF + Cu 3) Predict the products of the following reaction: K3N Goal(s): Write chemical reactions Molarity calculations and demonstrating proper lab technique Agenda: Do Now (show me page 143 & 170) 1) 1) Turn in Old, grab new one! 2) Finish Reaction Notes (Part C & we are going to do two book problems together) 3) HW Check 4) Pass Back Quizzes (Go over most missed q’s) 5) Ionic Compounds Lab HW: Pg. 176: 3a,c,d,g, j; 4a, d Make sure to have Pg 164: 4bd, 5c; - (that was on last time’s HW); Quiz Corrections HW Check! Pg. 166: 1) SR 2) S 3) C 4) DR 5) D Pg. 169: 1) Coefficients: 1, 3, 2; Synthesis 2) Coefficients: 1, 2, 1, 2; Combustion 3) 1, 1, 1; Decomposition Pg. 170 1) 2 Fe2O3 (s) → 4 Fe + 3 O2 – Decomp 2) 2 C3H7OH (l) + 9 O2 (g) → 6 CO2 (g) + 8 H2O (l) – C (this is a tough one to balance – look at the balancing tips!) 3) Ag2O (s) + H2O (l) → 2AgOH – Synth (an exception to the synthesis rule) 4) Pb(OH)4 PbO2 + 2H2O - Decomp (this is another tough decomp eq look at the back of the note sheet and the exceptions to the synth and decomp rxns) HW Check! Pg. 171 1. 3 Na (s) + AlCl3 (aq) → 3 NaCl (aq) + Al (s) 2. Cu (s) + KBr (aq) → No Reaction 3. F2 (g) + 2 LiI (aq) → 2 LiF (aq) + I2 (g) 4. Ca (s) + 2 HOH (l) → Ca(OH)2 (s) + H2 (g) Pg. 173 1) 3 HCl (aq) + Fe(OH)3 (s) → FeCl3 + 6 H2O – N 2) Zn (s) + 2 AgClO3 (aq) → 2 Ag + Zn(ClO3)2 – SR 3) (NH4)2S (aq) + 2 CsOH (aq) → Cs2S + 2 NH4OH - DR 4) 8 Cl2 (g) + 8 Li2S (aq) → 16 LiCl + 8 S- SR There were a few questions on solute/solvent… Solutions Video! Finding area in ft2. In order to use the 1 foot = 12 inches conversion, you must square it! 1 ft2 = 144 in2 Mass # = P + N (the atomic mass is the average mass of all the isotopes of that element) Ions = when an atom has either lost or gained electrons. This leads to an uneven P & E. Example: These are the isotopes of hydrogen (they are used in fusion reactions and Spiderman 2) – Hydrogen-3: has a mass of 3 and not 1 like the periodic table. Most hydrogen atoms are hydrogen-1(which is why the atomic mass is 1.01). 10/20/2015 1) Balance the following reaction: CH4 + O2 CO2 + H2O 2) Label the type of reaction: HF + NaOH H2O + NaF 3) Predict the products of the following reaction: Solid lithium reacts with fluorine gas. Goal(s): Write chemical reactions Demonstrate proper lab techniques with double replacement reactions Agenda: 1) Do Now (show me page 176) 2) Grade HW 3) HW Discussion Groups (18 mins) 4) Ionic Compounds Lab 5) Exit Ticket *Go over common quiz mistakes with B1 HW: Pg. 176: 1 a-f; 2a, 3 h-l; 4 b,c *Ionic Compounds lab and Pages 233-241 due 10/29 Pg 164 4b) 2, 3, 2 4d) 2, 3, 1, 6 5c) 2 N2 (g) + 6 PbO 2 Pb3N2 + 3 O2 (g) Pg. 176 3a) SR 2 Rb + ZnF2 2 RbF + Zn 3c) DR Pb(NO3)2 + 2 NaCl PbCl2 + 2NaNO3 5c) -1 point for the right reaction and elements, compounds written properly -1 point for the correct coefficients Problem 3 -1 point for right classification -1 point for right products -1 point for it balanced correctly 3d) D H2CO3 H2O + CO2 (complex decomp look at that complex chart) 3g) C; 2 C2H6 + 7 O2 4 CO2 + 6 H2O 3j) SR 3 Br2 + 2 InI3 2 InBr3 + 3 I2 4a) S; 6 Mg (s) + 2 N2 (g) 2 Mg3N2 4d) SR; Zn + CuSO4 Cu + ZnSO4 Total the number wrong on page 164. Total points: 25! Problem 4 -1 point for write classification -1 point for right elements/compounds written properly -1 point for it balanced correctly Amount Wrong Percentage 0-4 100 5-6 95 7-9 90 HW 2.5 10-12 85 Count up the amount of marks they have, 13-15 80 16-18 75 19-20 70 21 65 22 60 23 50 24 40 25 30 Uh so you didn’t do it 0 and use the table to the right for a score Blank spaces for assignments page count for a mark! Write score on top of pg. 164 Sometimes I just want to scream, but I don’t. Pg. 176 3a) SR 2 Rb + ZnF2 2 RbF + Zn 3c) DR Pb(NO3)2 + 2 NaCl PbCl2 + 2NaNO3 3d) D H2CO3 H2O + CO2 Problem 3 -1 point for right classification -1 point for right products -1 point for it balanced correctly (complex decomp look at that complex chart) 3g) C; 2 C2H6 + 7 O2 4 CO2 + 6 H2O 3j) SR 3 Br2 + 2 InI3 2 InBr3 + 3 I2 Total the number wrong on page 176. Total points: 19! 4a) S; 6 Mg (s) + 2 N2 (g) 2 Mg3N2 4d) SR; Zn + CuSO4 Cu + ZnSO4 Problem 4 -1 point for write classification -1 point for right elements/compounds written properly -1 point for it balanced correctly Amount Wrong Percentage 0-3 100 4-6 95 5-7 90 HW 2.5 8-10 85 Count up the amount of marks they have, 11-12 80 13 75 14 70 15 65 16 60 17 50 18 40 19 30 Uh so you didn’t do it 0 and use the table to the right for a score Blank spaces for assignments page count for a mark! Write score on top of pg. 176 Sometimes I just want to scream, but I don’t. Grab a markerboard and marker! -18 minutes 1) Go over today’s HW problems. Make sure every group member understands them! 2) Go over any Chapter 3 problems if you have time. 3) Get lab sheet out when done! Lab Safety! WEAR YOUR DARN SAFETY GLASSES >:( >:( Share the set of chemicals with the group across from you!! (Possibly split set into two and then switch after testing all those chemicals) 1. Make sure you know what chemical you put in each well plate. 2. Record your observations Cloudiness, Color, Texture If you need more space to record, flip it over and just record Reaction # 3. Make sure you clean your well plate and show me for initials! 4. For analysis: Make sure to look at the examples! You can use the back space of the papers (since I messed up and didn’t do double-sided) for your answers! 1) Write the complete balanced reaction of: Na2O -After you finish put your pen/pencil away, and get a highlighter, different color or a marker from the side table. Put a for a correct answer Put a for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! 1) 2 Na2O 4 Na + O2 10/22/2015 1) Balance the following reaction: C5H5 + O2 CO2 + H2O 2) Label the type of reaction: Na2SO4 + 2 Cu Cu2SO4 + 2 Na 3) Predict the products of the following reaction: Br2 + InI3 In the early 1900’s the government wanted people to drink less alcohol, so manufacturers put deadly poisons including: iodine (and a whole bunch of others in it). Goal(s): Write chemical reactions Review Mole Conversions Agenda: 1) Do Now (show me page 164 and 176) 2) Grade HW 3) HW Discussion Groups (20 mins) Finish lab if needed 4) Review Game 5) Review Sheet 6) Exit Ticket In 1926, in New York City, 1,200 were sickened by poisonous alcohol; 400 died. HW: Review Sheet, Quiz next class The following year, deaths climbed to 700. *Ionic Compounds lab and Pages 233-241 due Pg 176 1a) DR 1,2,1,2 1b) SR 1,2,1,2 1c) S 2,3,2 1d) D 2,1,3 1e) SR 1, 2, 1, 1 1f) DR 2, 3, 1, 6 3h) SR 2 Cs + NiCl2 2 CsCl + Ni 3i) D Zr(OH)4 2 H2O + ZrO2 We already graded 3j 1 point for each question! 3k) N (or DR) 2 H3PO4 + 3 Ba(OH)2 6 H2O + Ba3(PO4)2 3l) DR 2 AgNO3 + Ca(CH3COO)2 Ca(NO3)2 + 2 AgCH3COO 4b) N (or DR); H3PO4 (aq) + Fe(OH)3 3 H2O (l) + FePO4 4c) C; 2 C4H10 (g) + 13 O2 (g) 8 CO2 (g) + 10 H2O (g) Total points: 12! Total the number wrong on page 177. Only one thing wrong with the problem? Just take a half off instead of one HW 2.7 Count up the amount of marks they have, and use the table to the right for a score Blank spaces for assignments page count for a mark! Write score on top of pg. 177 Good luck on the ACT! Remember when in doubt, Amount Wrong Percentage 0 to 2 100 3 95 4-5 90 6-7 85 8 80 9 75 10 70 65 11 60 12 50 40 30 Uh so you didn’t do it 0 Grab a markerboard and marker! -20 minutes 1) Go over today’s HW problems. Make sure every group member understands them! 2) Go over any Chapter 3 problems if you have time (that will be what our quiz is about!) 3) Make sure collecting lab data is done! -Send one member from your group to get: A marker, markerboard and a tissue -The first group will choose the category + point value -Every group writes a response down! -Mr. P will say BOARDS UP, and each team puts their board up How many grams of oxygen are there in a 4 liters of oxygen at STP? Hint #1: Is oxygen diatomic? Hint #2: Start with what you are given and work your way! Hint #3: Uh it’s a chemistry problem -After you finish put your pen/pencil away, and get a highlighter, different color or a marker from the side table. Put a for a correct answer Put a for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! 1) 5.71 g O2 10/27/2015 1) Get Review Sheet out (Turn in) 2) Study! Ask questions! Check out answer key if you have not already! Goal(s): Write chemical reactions Assess over Mole Conversions Agenda: 1) Do Now 2) Stoich Problem 3) Mole Quiz 4) Solubility Examples 5) Finish & Turn in I. Compounds When done, can work on HW Pages 6) Exit Ticket HW: Ionic Compounds Lab Due 10/29 Pages 233-241 due 11/2 Q7 on review sheet This method works best when you have TWO DIFFERENT SUBSTANCES! The mole is what let’s you relate them! What mass of carbon is present in 4.8x1025 molecules of ethanol (C2H5OH)? This method works best when you have TWO DIFFERENT SUBSTANCES! The mole is what let’s you relate them! How many liters of fluorine are in 30 grams OF3? -Be respectful of your classmates and STAY QUIET -When finished: -Double check your work! -Bring it up and turn it into the front -Pick up your review sheet -Work on: -Get Lab Out (we will go over two things) -Start on homework -IR book Key points: -All nitrates are soluble -Compounds that have alkali metals are soluble *Soluble = dissolve in water = (aq) *Insoluble = does not dissolve in water = (s) – it’s a precipitate! Let’s look at two examples! ZnCl2 (aq) + Pb(NO3)4 (aq) Fe(NO3)3 (aq) + BaCl2 (aq) -Finish the analysis section of the Ionic Compounds Lab and turn in! - Use Solubility Chart!! -Finished early? Can start on HW or read IR Book 1. Write the balanced double replacement reaction of: CaI2 (aq) + KOH (aq) 2. Write the net ionic equation of the previous problem! -After you finish put your pen/pencil away, and get a highlighter, different color or a marker from the side table. Put a Put a for a correct answer for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! 1) CaI2 (aq) + 2 KOH (aq) Ca(OH)2 (s) + 2 KI (aq) 2) Ca+2 (aq) + 2 OH- (aq) Ca(OH)2 (s) 10/29/2015 1) Is KNO3 soluble (aq) or insoluble (s)? 2) What state would Pb(OH)2 be in? Hint: You may need Solubility chart for 1&2 3) Write the balanced equation: Nitrogen reacting with Hydrogen to form Nitrogen Trihydride Hint: Is N or H diatomic? This is in your book on page 234 #1! Write it in! Goal(s): Convert molar quantities between reactants and products Agenda: 1) Do Now (show me page: 176 for old graded HW) 2) Calcium Carbide Demo 3) Stoichiometry Tips 4) Challenge #3 with Lab Group 5) HW Work Time 6) Exit Ticket HW: Pages 233-239 2H2 (g) + O2 (g) 2H2O (g) How many liters of oxygen gas at STP would need to completely react with 10 grams of hydrogen? -After you finish put your pen/pencil away, and get a highlighter, different color or a marker OUT!! -If you do not have a highlighter, wait until I say to go grab one! Put a Put a for a correct answer for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! 56 L O2 work shown 11/2/2015 1) How many moles of C2H4 are in 15.5 grams of C2H4? 2) How many grams of C2H4 are in 30.5 L at STP? If you have: -Alchemist Lab -Blue Lab -Green Lab Turn into me! Or I will choose cation and use maul! Goal(s): Convert molar quantities between reactants and products using molarity Agenda: 1) Do Now (show me page: 237) 2) Stoichiometry Notes Cont. 3) HW Check 4) HW Discussion Groups 5) Challenge #3 with Lab Group 6) Exit Ticket HW: Pages 240-241 & 243:#1 Pg. 246-249 & Pg 243: 2, 3, 7, 9 Due 11/9 Pg 233 (W-UP): 1) 2 Cr (s) + 6 HCl (aq) 2 CrCl3(aq) + 3 H2 (g) A lot of these depend on having the correct 2) 3 mol H2 3) 6 mol H2 balanced reaction! Pg 234: 1) 31.5 mol NH3 3) 0.93 mol Ca 2) 1.89x10-4 mol H2O Pg. 236: 1) 17.9g O2 2) 11,000,000 g (or 1.1 x 107) 3) 4.263 mol B 4) 2 Na(s) + H2O(l) Na2O + H2 (g); 1 mol H2 gas; 22.4 L Pg. 237 (QC): 30 L CO2 Pg 239: 1) 1.21x107 L O2 2) 13.8 L 3) Zn3As2(aq) + 6HCl(aq) 2AsH3(g) + 3ZnCl2(aq); 85.5 g Without it, it would be impossible to do! In your groups make sure you have the correct equation. N2 + 3 H2 2 NH3 2 H2O 2 H2 + O2 Ca (s) + 2 AgNO3 Ca(NO3)2 + 2 Ag 2, 6, 2, 3 3 H2 + 2 BCl3 2 B + 6 HCl 2, 1, 1, 2, 2 Zn3As2 + 6 HCl 2 AsH3 + 3 ZnCl2 *these last two made me scratch my head for a sec Grab a markerboard and marker! 1) Go over today’s Reading problems. Make sure every group member understands them! I will leave answer slide up with coefficients for eq and reactants/productes written properly! 2) When finished going over the Reading Problems, start Challenge #3 Sheet! (for the last one find it in moles, g, L and particles!) How many liters of water vapor at STP would be produced if 2.5 x 1022 particles of oxygen reacts with an excess amount of hydrogen gas? -After you finish put your pen/pencil away, and get a highlighter, different color or a marker OUT!! -If you do not have a highlighter, wait until I say to go grab one! Put a Put a for a correct answer for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! 1.86 L H2O work shown 11/4/2015 1) Write the equation between solid aluminum reacting with fluorine gas. 2) How many liters of fluorine gas at STP are needed to react with 10.0 grams of aluminum? Goal(s): Convert molar quantities using the limiting reactant Agenda: 1) Do Now (show me page: 241) 2) Stoichiometry Notes Cont. 3) HW Check/HW Discussion Groups 4) Limiting Reactants Simulation Activity 5) AP Chem Day 11/14? 6) Exit Ticket HW: Pg. 246-249 & Pg 243: 2, 3, 7, 9 Pg. 250-252 & Pg 255: 1-3, 7, 14 Due 11/12 Pg 240 (these questions use Molarity equation) 1) 1.3 M 2) 19 g 3) 0.51 L Without #1 right, your answers for #2 and #3 will be off. Hopefully you got the process! Pg 241: 1) 4.4 g KOH 2) 10 L NaI (rounded from 9.97) 3) 88 L CO2 Page 243 #1, we will grade on Monday. It’s a regular stoichiometry problem, only tricky thing is getting the molar mass of that bad boy malachite *Malachite sounds like a pokemon. *Remember this symbol • just means it contains it; all those substances get added up for the molar mass. Just add them regularly! Grab a markerboard and marker! 1) Go over pg. 240-241. Make sure every group member understands them! I will leave answer slide up on the computer 2) When finished going over the Reading Problems, start Limiting Reactants Simulation (come receive a chromebook) http://goo.gl/forms/gOMX03TjpB Still working out details if we can join the other Noble schools over at Munich But I made copies of a permission slip; go to that link regardless whether you can make it 11/14 It would be 9:30 AM to 12:30 PM – we would meet 9:00 AM here and then head together over there 1 N2 (g) + 3 H2 (g) 2 NH3 (g) If 20.5 grams of nitrogen reacts with 5.0 grams of hydrogen, how many liters of ammonia at STP can you produce? What is the limiting reactant? -After you finish put your pen/pencil away, and get a highlighter, different color or a marker OUT!! -If you do not have a highlighter, wait until I say to go grab one! Put a Put a for a correct answer for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! Nitrogen is the limiting reactant 32.8 L NH3 work shown 11/9/2015 1) How was your weekend? 2) Based on the following UNBALANCED reaction, how many grams of N2 would be produced when 30 grams of NH3 is decomposed? NH3 N2 + H2 Goal(s): Convert molar quantities using the limiting reactant Agenda: 1) Do Now (show me page: 248-49) 2) Finish Stoich Notes (15 mins) 3) Limiting Reactants Simulation Activity 4) Quiz Back In-Class Retake on Monday 11/16 HW: Pg. 250-252 & Pg 255: 1-3, 7, 14 Review Sheet due 11/18 Important Dates: First Test: 11/18 – Reactions & 1) Receive a Chromebook 2) Go to the phet.colorado! 3) Use the simulation to review limiting reactants! 4) When you finish working through the last two pages, get the answers checked in the cabinet door! One mistake on the back! This should be 2 mol H2S Grab a markerboard and marker! 1) Go over pg. 243, 246-9. Make sure every group member understands them! 2) Pick Up Quizzes Quiz Tip Slide will be posted up 11/12/2015 1) Balance the reaction below! What type of reaction is it? 2) Based on the following UNBALANCED reaction, how many liters of O2 at STP would be produced when 25 grams of H2O is decomposed? Goal(s): Convert molar quantities relating reactants and products Agenda: 1) 2) Do Now (show me page: 243, 251-252) Announcements 3) Grade HW/HW Check (15 minutes) 4) Challenge #3 (35 minutes) This reviews the quiz material we H2O H2 + O2 got back. Preps for optional in-class retake on 11/16 (you must correct your original quiz!) 5) Exit Ticket (10 minutes) HW: Quiz Corrections, Study for Quiz Retake Review Sheet due 11/18 Important Dates: First Test: 11/18 – Reactions & Stoich. 1) Go here (this was emailed out): http://goo.gl/forms/gOMX03TjpB This signs you up officially You can swing by here after school and you can sign up using my computer 2) Dress Code: khakis, sneakers of the kids’ choice (closed toe), and Rauner polo. Location: You can just go straight there! I think that’ll be easier for everyone; plan to meet there! Arrive between 9:15 AM to 9:30 AM 3) Right now, the bonus is 249.5 extra credit points Seems like we had a lot of calculating mistakes with Avogadro’s number even though all the work is right Store 6.022 x1023 in your calculator Type 6.022E23 (For TI-83: 2nd + EE) Once you have that typed on your calculator, find the STO button and click it, now click ALPHA and then A or ALPHA and then M. Depending if you want to store it under Avogadro or Molecules. Now we can just hit ALPHA + A and get Avogadro’s, instead of typing it out everytime. 1) Part of the Retake will be given in class on Monday. You must correct your original quiz! This will be used to replace those type of questions These will be the mole quantity conversion ones 2) The other part of the retake is during office hours Empirical Formula/Molecular Formula, Percent Composition, etc Come to office hours in order to be eligible for this portion! 1) Working with your lab group, solve the set of questions given to you 2) After each section, get it checked by the teacher and signed off! 3) Move onto the next section! 4) Last question, assume at STP! -If you finish early, make sure you finish Limiting Reactants Simulation problems and have it checked! Pg 246-249 Pg 246: 1) 4 Sundaes 2) Syrup 3) 471 cherries, 2 scoops Page 247: 1) Oxygen 3) 4 4) 1 O2 Page 248: 1) 0.694 g H2 ER: Zn by 2.6 g 2) 4.4 g H2O ER: 59.4 g NaHCO3 3) 56.25 g CaH2 ER: 53.6 g Ca Page 249: 1a) 84.4% 1b) 87.5% Page 248 is the trickiest: 1) Find the LR that limits the product 2) See how much of the excess reactant gets used up by the limiting. Subtract the left over. 3) 0.81 g Pg 250-252 Pg 251: 1) 4.38 g Al2O3 2) 47.95 g Fe2O3 3) 98.6% Pg 252: Theoretical = what you SHOULD get (pure) the amount you get through stoichiometry! 1) 87.1% 2) 92.6% 3) 13.9 g CuO WATCH THE VIDEOS ON % PURITY & % YIELD!! It’s a tricky topic! Pg 243: 1-3, 7, 9 1) 2.52 mol CuO 1b) 1106 g 1c) 97.0 L 2a) 580 L 2b) 4160 g 2c) 3100 g 3) 0.15 g Zn 7) 0.017 mol H2SO4 -9 points on Page 243 9) 84.3 g FeS -Must Have work for credit! HW 3.8 Amount Wrong Percentage 0-2 100 3 95 4 90 5 85 Count up the amount of marks they have, and use the table to the right for a score Blank spaces for assignments page count for a mark! Write score on top of pg. 243 They say there’s a lot of fish in the sea, but I don’t seA it sometimes.. 80 6 75 7 70 65 8 60 9 50 40 30 Uh so you didn’t do it 0 11/16/2015 1) How was your weekend? 2) Based on the following UNBALANCED reaction, how many liters of O2 would be produced when 25 grams of H2O is decomposed? Goal(s): Convert molar quantities relating reactants and products Agenda: 1) 2) 3) 4) Do Now (show me page: 243, 251-252) Announcements Grade HW/HW Check (15 minutes) Challenge #3 (35 minutes) This reviews the quiz material we got back. Preps for optional in-class retake on 11/16 (you must correct your original quiz!) H2O H2 + O2 HW Disc Group (20 minutes) Finish Limiting Reactants Simulation Post Q’s; check your answers and show teacher 7) Exit Ticket (10 minutes) 5) 6) HW: Quiz Corrections, Study for Quiz Retake Review Sheet due 11/18 Important Dates: First Test: 11/18 – Reactions & Stoich. Pg 255: 1-3, 7, 14 1) nachooooooo 2) 3) 7) 3.56 g 14) 5.02 L 1 N2 (g) + 3 H2 (g) 2 NH3 (g) If 10 grams of Nitrogen gas reacted with an excess amount of hydrogen and produced 10 liters of ammonia, what is the percent yield of the reaction? -After you finish put your pen/pencil away, and get a highlighter, different color or a marker OUT!! -If you do not have a highlighter, wait until I say to go grab one! *Turn in Exit Ticket Put a Put a for a correct answer for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! 62.5% Work is shown --> HW 3.8 Amount Wrong Percentage 0-2 100 3 95 4 90 5 85 Count up the amount of marks they have, and use the table to the right for a score Blank spaces for assignments page count for a mark! Write score on top of pg. 243 Sometimes I just want to scream, but I don’t. 80 6 75 7 70 65 8 60 9 50 40 30 Uh so you didn’t do it 0 Mole Quiz Retake You must correct your original quiz! This will be used to replace those type of questions These will be the mole quantity conversion ones This will be an optional Wednesday! section of the test on 11/16/2015 1) How was your weekend? 2) Label the following reactions as S, D, SR, DR, C, N: (a) (b) (c) (d) (e) Goal(s): Convert molar quantities relating reactants and products Agenda: 1) Do Now 2) 3) 4) 5) Overview of the format of the test Grade HW/HW Check Finish Challenge #3 Study Guide: Aluminum Foil Activity Finish Limiting Reactants Simulation Questions and check answer in cabinet door Optional Retake in class is moved unless we have extra class time here 6) Exit Ticket (10 minutes) HW: Quiz Corrections, Review Sheet Important Dates: First Test: 11/18 – Reactions & Stoich. Pg. 2 due 11/20 25-30 Multiple Choice Questions 3 FRQ AP Questions AP Test Curved 75% M.C. & 60% F.R.Q. 5 100% Note: on the AP test, you will not know everything, get used to seeing questions you may not know, so there may be 2 or 3 multiple choice questions that you have not seen, do your best! Note #2: On the M.C. of the real AP test, you will not be able to use a calculator, but on the FR you will. There may be some empirical formula questions! Bonus Question Working with your lab group, solve the set of questions given to you 2) After each section, get it checked by the teacher and signed off! 3) Move onto the next section! 1) 4) Last question, assume at STP! -Once finished, do the Study Guide Activity; first you figure out how to make the solution, then you will need: aluminum foil, a beaker, graduated cylinder, balance and copper (II) sulfate 1) Solve how you would make the solution indicated in the first part. 2) Have it checked by your teacher 3) Once you are ready to make the solution, Have your safety glasses on! Use lab equipment to carry out your procedure Answer the questions at the end Talk to your teacher at the end about what you think was happening For clean up, put the “gunk” into the LARGE beaker, and then rinse out your beaker. Make sure your area is clean! -Once finished, finish the Limiting Reactants Activity from last week, check your answers on the cabinet. And show your instructor! -If you still have time before Exit Ticket, go over any HW problems/work on Study Guide Questions! 1 N2 (g) + 3 H2 (g) 2 NH3 (g) If 20.5 grams of nitrogen reacts with 5.0 grams of hydrogen, how many liters of ammonia at STP can you produce? What is the limiting reactant? -After you finish put your pen/pencil away, and get a highlighter, different color or a marker OUT!! -If you do not have a highlighter, wait until I say to go grab one! Hint: see how many liters of ammonia you can produce from 20.5 g of Nitrogen and see how many liters of ammonia you can produce from 5 grams of hydrogren (set up two equations!) *Turn in Exit Ticket Put a Put a for a correct answer for an incorrect answer, but attempted (work is there) Put a for a blank answer (no attempt) *This should be done in a different color! Nitrogen is the limiting reactant 32.8 L NH3 sample work shown 11/16/2015 1) Take 8 minutes to study/ask questions/check study guide answers Hope ya’ll summon your inner Gandalf Goal(s): Assess over converting molar quantities relating reactants and products Agenda: 1) Do Now 2) Reactions & Stoichiometry Test 3) Calculate Oxidation States Video Time Permitted! This goes into our next unit: Electrochemistry and covers your book reading for Friday HW: Pg. 2 Book 2: Pg. due 12/1 -Be respectful of your classmates and STAY QUIET -When finished: -Double check your work! -Bring it up and turn it into the front -Pick up your review sheet -Work on: -Start on homework -IR book