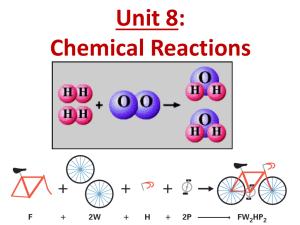
20u2 Chapter 6.5 - Balancing Chemical Equations
advertisement

BALANCING CHEMICAL EQUATIONS Chapter 6.5 DAY 1 & 2 Word equations or formula (skeleton) equations need to be balanced. The number of atoms of one element on the reactant side of an equation must equal the number of atoms of that same element on the product side of an equation. LAW OF CONSERVATION OF MASS States: the total mass of atoms must be the same on both sides of an equation. Meaning –> the mass of the reactants equals the mass of the products. The same total number of each atom = same total atomic mass LETS BALANCE EQUATIONS STEP 1 – Write the formula equation of the reaction. Example: CH4 + O2 CO2 + H2O STEP 2 – Count the number of atoms of each type on the reactants side and on the products side reactants O–2 C–1 H–4 products O-3 C-1 H–2 3) Multiply each of the formulas by an appropriate coefficient to balance the numbers of atoms CH4 + 2 O2 CO2 + 2 H2O 4) Count atoms on each side to verify. reactants products O–4 O-4 C–1 C-1 H–4 H–4 GROUP PRACTICE 1) hydrogen and oxygen produce water 2) calcium and chlorine make calcium chloride 3) aluminum and oxygen produce aluminum oxide 4) copper and iron(III) oxide make iron and copper(I) oxide HOMEWORK PAGE 229 # 2, 3ade Practice worksheet BLM 6.5c Assignment