double replacement lab
advertisement
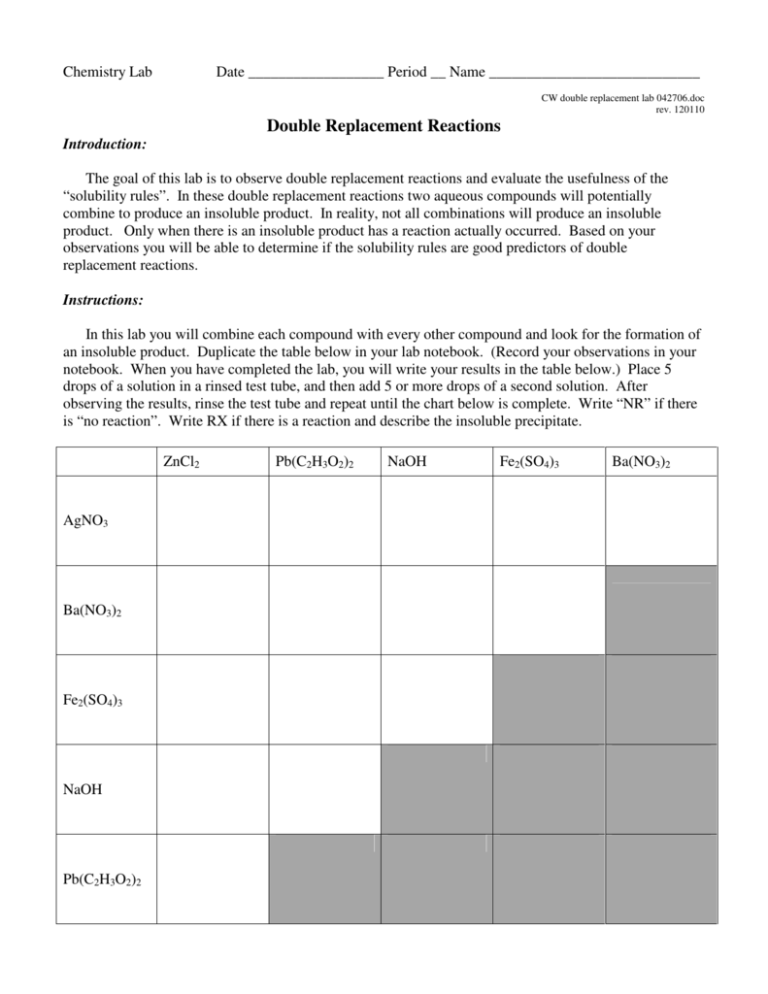
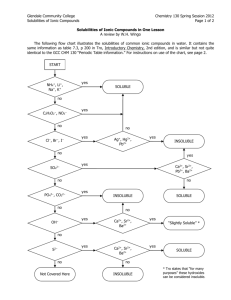
Chemistry Lab Date __________________ Period __ Name ____________________________ CW double replacement lab 042706.doc rev. 120110 Double Replacement Reactions Introduction: The goal of this lab is to observe double replacement reactions and evaluate the usefulness of the “solubility rules”. In these double replacement reactions two aqueous compounds will potentially combine to produce an insoluble product. In reality, not all combinations will produce an insoluble product. Only when there is an insoluble product has a reaction actually occurred. Based on your observations you will be able to determine if the solubility rules are good predictors of double replacement reactions. Instructions: In this lab you will combine each compound with every other compound and look for the formation of an insoluble product. Duplicate the table below in your lab notebook. (Record your observations in your notebook. When you have completed the lab, you will write your results in the table below.) Place 5 drops of a solution in a rinsed test tube, and then add 5 or more drops of a second solution. After observing the results, rinse the test tube and repeat until the chart below is complete. Write “NR” if there is “no reaction”. Write RX if there is a reaction and describe the insoluble precipitate. ZnCl2 AgNO3 Ba(NO3)2 Fe2(SO4)3 NaOH Pb(C2H3O2)2 Pb(C2H3O2)2 NaOH Fe2(SO4)3 Ba(NO3)2 Data Analysis: 1. Determine the compounds which we know from the beginning are soluble in water. 2. On notebook paper, write a balanced molecular equation for each combination where a “no reaction” occurred. This will enable us to expand our list of compounds which are soluble in water. The “products” of every “no reaction” are soluble in water. Write the formulas of the soluble compounds in the boxes below. 3. For each combination where a precipitate formed there was an insoluble product. Write a balanced molecular equation for each and use your list of known soluble compounds to predict the insoluble product. Write the formulas of the insoluble compounds in the boxes below. Summary – Write the results of your observations in the appropriate boxes List the water soluble compounds grouped by anion Acetate C2H2O2 - Acetate C2H2O2 - Chloride Cl- Hydroxide OH- Nitrate NO3- Sulfate SO42- List the insoluble compounds grouped by anion Chloride Cl- Hydroxide OH- Nitrate NO3- Sulfate SO42- Chemistry Worksheet Date __________________ Period __ Name _____________________________ CW double replacement lab 042706.doc rev. 112910 Double Replacement Reactions and the Solubility Rules Compare the compounds on your lists of soluble and insoluble compounds on your summary page to the predictions made by the solubility rules which are found in your NCDPI Reference Tables, and reprinted below. Part 1. Answer these questions on notebook paper. 1. What is the “indicator” that a double replacement reaction has occurred? 2. Consider the case where solutions of calcium nitrate and aluminum sulfate are combined. a. Predict the possible products (write the correct formulas) as if a reaction occurred. b. Using the solubility rules determine if a possible product is insoluble. c. Write the balanced chemical equation and include state symbols. 3. How could you determine if a double replacement reaction occurs without actually doing the reaction? 4. What might account for the differences between your observations and those predictions based by the solubility rules? For example, why do the rules predict that PbCl2 is insoluble, but you might not observe a precipitate when Pb(C2H3O2)2 and ZnCl2 were mixed? 5. A precipitate will form when solutions of potassium hydroxide and copper (II) nitrate are mixed. Write the balanced molecular equation for the reaction and include state symbols. 6. Write the balanced net ionic equation for the reaction described above. 7. In a similar experiment, students wanted to know if strontium sulfate is soluble or insoluble. Of course the solubility rules predict that strontium sulfate is insoluble in water. What would be the best choice for two chemicals that they could mix so that any precipitate that formed would be solely attributable to strontium sulfate? 8. Using the solubility rules, write balanced molecular and net ionic equations for the reaction between solutions of copper (II) nitrate and sodium phosphate. Be sure to include state symbols in the molecular equation. Part 2. Determine if a reaction occurs, write and balance the molecular equation including state symbols, then write the net ionic equation below it. If no reaction occurs, write NR. If the equation is balanced as written, write BAW. Answer either on this sheet or on notebook paper. 1. Na2SO4(aq) + LiCl(aq) → 2. CuCl2(aq) + AgNO3(aq) → 3. Sr(OH)2(aq) + 4. Fe2(SO4)3(aq) → AlCl3(aq) + Zn(C2H3O2)2(aq) → 5. K2CO3(aq) + NiCl3(aq) → 6. KI(aq) + 7. Na3PO4(aq) + 8. HCl(aq) + 9. H2SO4(aq) + 10. Pb(NO3)2(aq) + 11. CuBr2(aq) + 12. Bi(NO3)3(aq) + 13. AuCl3(aq) + LiOH(aq) → 14. FeBr2(aq) + Al2(SO4)3(aq) → 15. (NH4)2S (aq) + Hg2(NO3)2(aq) → SnCl2(aq) → NaOH(aq) → Ba(OH)2(aq) → Na2S(aq) → Fe(C2H3O2)3(aq) K3PO4(aq) → ZnCl2(aq) → →