File
advertisement
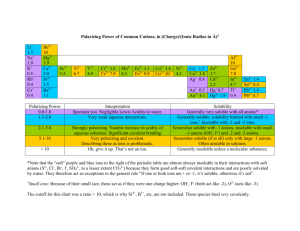
Unit 10: Energy in Chemical Reactions Cypress Creek High School Chemistry 1K Chapter 10 Solubility Rules • We will use a solubility chart to determine if chemicals will be: – Soluble in water and considered aqueous (aq) or… – Insoluble in water and be considered precipitates/solids (s) • The solubility chart lists ions (single or multiple elements that have a collective charge) and their solubilities. These ions will be written with a +/ – and a number written as a superscript. – Ion Examples… • • • • Ca2+ is the element calcium’s ion F– is the element fluorine’s ion SO42- is a polyatomic ion made up of sulfur & oxygen named sulfate NH4+ is a polyatomic ion made up of nitrogen & hydrogen named ammonium Solubility Rules • The chart also mentions alkali metal cations, which include the following elements in the first column of the periodic table: – Li+ – Na+ – K+ – Rb+ – Cs+ – Fr+ Any compound not listed in these general rules is considered insoluble. Solubility Rules Chart Any compound that contains these ions is always soluble Any compound that contains these ions is always soluble except with the ions listed Any compound that contains these ions is never soluble except with the ions listed Solubility Practice • Practice using the solubility rules for the following compounds: – Ca(NO3)2 (aq) Soluble: contains NO3– AgCl (s) Insoluble: Cl- is soluble, unless paired with Ag+, Pb2+, or Hg22+ – Ni(OH)2 (s) Insoluble: OH- is insoluble, unless paired with NH4+, the alkali metal cations, Ca2+, Sr2+, or Ba2+ Combustion Reaction • Whenever hydrocarbons combine rapidly with oxygen (usually by fire), the reaction is called a combustion reaction. – The products will be water and carbon dioxide. – Burning methane (CH4) yields water and carbon dioxide • This reaction contributes to our greenhouse gases and global warming. Identifying Chemical Reactions • Identify each of the following chemical equations as synthesis, decomposition, single-displacement, double-displacement, or combustion reaction. A) B) C) Predicting Chemical Reactions Steps 1. Determine what type of reaction is being presented 2. Write the correct formulas for the product(s) 3. Balance the equation Example: CaCl2 + Al(OH)3 ? 1. Double-displacement reaction 2. __CaCl2 + __Al(OH)3 __Ca(OH)2 + __AlCl3 3. 3CaCl2 + 2Al(OH)3 3Ca(OH)2 + 2AlCl3 Predicting Chemical Reactions Practice • Predict the chemical reaction: NaCl (aq) + Pb(NO3)2 (aq) ? 2NaCl (aq) + Pb(NO3)2 (aq) PbCl2 (s) + 2NaNO3 (aq) • Predict the chemical reaction: Mg (s) + CuSO4 (aq) ? Mg (s) + CuSO4 (aq) MgSO4 (aq) + Cu (s)