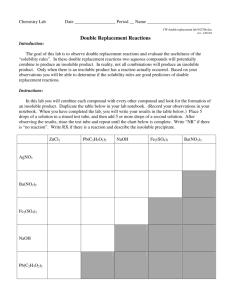
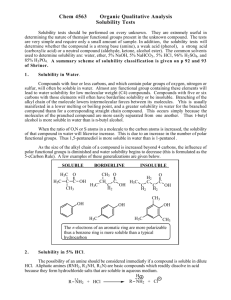
Solubiility Worksheet

Name: _________________________________________ Block: ___________ Date: ______________
Solubility Worksheet
Directions : Use the SOLUBILITY RULES section of your Chemistry Reference Tables (p.6) to help you answer the following:
1.
Circle the following compounds that are soluble in water.
NaOH Fe
2
S
3
MgSO
4
PbCl
2
Ba(NO
3
)
2
MgCO
3
2. Predict whether each of the following compounds is soluble or insoluble in water. a) Magnesium Phosphate g) Li
2
CO
3 b) Silver (I) Nitrate h) NH
4
Br c) Barium Carbonate i) Ni(NO
3
)
2 d) Calcium Chloride j) PbSO
4 e) Aluminum Sulfide k) FeCl
3 f) Na
2
SO
4 l) Na
3
PO
4
2. Write a balanced equation to show the dissociation of each of the following compounds in water. a) Na
2
S b) Ca(NO
3
)
2 c) Magnesium sulfate d) K
3
PO
4
________________________________________________________
________________________________________________________
________________________________________________________
________________________________________________________ e) Ammonium carbonate ________________________________________________________ f) Cu(OH)
2
________________________________________________________
Name: _________________________________________ Block: ___________ Date: ______________
Solubility Worksheet
Directions : Use the SOLUBILITY RULES section of your Chemistry Reference Tables (p.6) to help you answer the following:
2.
Circle the following compounds that are soluble in water.
NaOH Fe
2
S
3
MgSO
4
PbCl
2
Ba(NO
3
)
2
MgCO
3
2. Predict whether each of the following compounds is soluble or insoluble in water. a) Magnesium Phosphate g) Li
2
CO
3 b) Silver (I) Nitrate h) NH
4
Br c) Barium Carbonate i) Ni(NO
3
)
2 d) Calcium Chloride j) PbSO
4 e) Aluminum Sulfide k) FeCl
3 f) Na
2
SO
4 l) Na
3
PO
4
2. Write a balanced equation to show the dissociation of each of the following compounds in water. a) Na
2
S b) Ca(NO
3
)
2 c) Magnesium sulfate d) K
3
PO
4
________________________________________________________
________________________________________________________
________________________________________________________
________________________________________________________ e) Ammonium carbonate ________________________________________________________ f) Cu(OH)
2
________________________________________________________