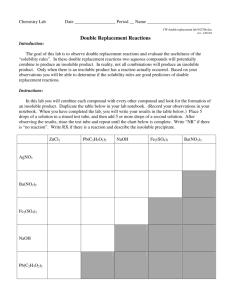
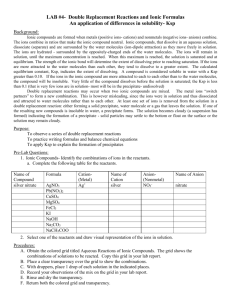
Ionic Compound Solubilities in One Lesson Flow Chart
advertisement
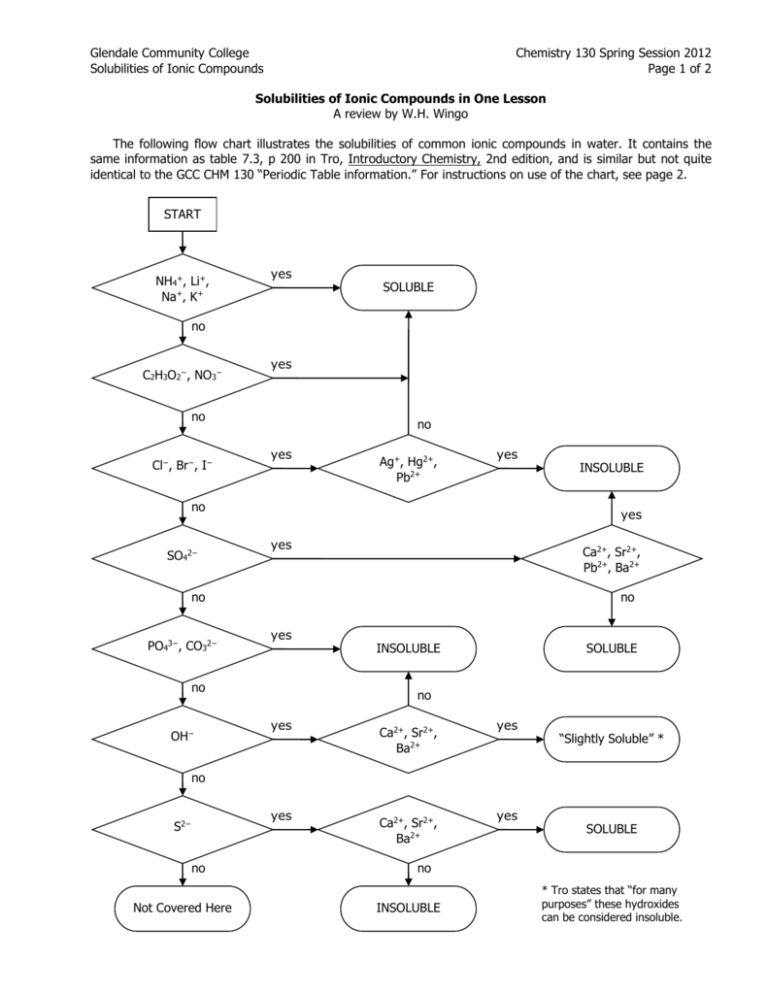
Glendale Community College Solubilities of Ionic Compounds Chemistry 130 Spring Session 2012 Page 1 of 2 Solubilities of Ionic Compounds in One Lesson A review by W.H. Wingo The following flow chart illustrates the solubilities of common ionic compounds in water. It contains the same information as table 7.3, p 200 in Tro, Introductory Chemistry, 2nd edition, and is similar but not quite identical to the GCC CHM 130 “Periodic Table information.” For instructions on use of the chart, see page 2. START NH4+, Li+, Na+, K+ yes SOLUBLE no C2H3O2−, NO3− yes no Cl−, Br−, I− no yes Ag+, Hg2+, Pb2+ yes no SO42− yes yes Ca2+, Sr2+, Pb2+, Ba2+ no PO43−, CO32− no yes no OH− INSOLUBLE INSOLUBLE SOLUBLE no yes Ca2+, Sr2+, Ba2+ yes “Slightly Soluble” * no yes S2− no Not Covered Here Ca2+, Sr2+, Ba2+ yes SOLUBLE no INSOLUBLE * Tro states that “for many purposes” these hydroxides can be considered insoluble. Glendale Community College Solubilities of Ionic Compounds Chemistry 130 Spring Session 2012 Page 2 of 2 Use of the Flow Chart First we identify the correct formula of the ionic compound whose solubility is to be determined. Thus, if we are given “iron (II) sulfate,” we would first have to express it as FeSO4. Then we enter the chart at the START box and work our way down the left-hand column until we encounter an ion that is in our formula. For FeSO4, our first “yes” answer comes at sulfate, four boxes down. (Iron is not listed anywhere in the chart.) The “yes” answer for sulfate takes us to the decision box at the far right of the same line, and we must check our formula for calcium, strontium, lead (II), and barium ions. Since iron (II) is not listed, our answer here is “no,” which leads to the SOLUBLE outcome immediately below: iron (II) sulfate is soluble. Another example: Lithium carbonate is soluble because it contains lithium ion; however, any carbonate with a positive ion not listed in the first decision box, such as calcium carbonate or copper (II) carbonate, would give “no” answers until the fifth decision box down on the left. Here we learn that any carbonate not eliminated in the first decision box is INSOLUBLE. Exercises: Using the flow chart, classify each of the following ionic compounds as soluble or insoluble in water. 1. copper (I) chloride _______________________________ 2. germanium nitrate _______________________________ 3. sodium azide _______________________________ 4. potassium metabisulfate _______________________________ 5. lead (II) acetate _______________________________ 6. any transition metal sulfide _______________________________ 7. francium astatide (very rare….) _______________________________ 8. mercury (II) bromide _______________________________ 9. any transition metal sulfate _______________________________ 10. any column 2 phosphate _______________________________ 11. any transition metal hydroxide _______________________________ 12. tin (II) bromide _______________________________ 13. silver fluoride _______________________________ 14. lead (IV) acetate _______________________________ 15. potassium thiosulfate _______________________________ 16. barium sulfide _______________________________ 17. barium hydroxide _______________________________ 18. calcium nitrate _______________________________ 19. lead (IV) iodide _______________________________ 20. tin (II) phosphate _______________________________ 21. arsenic trioxide _______________________________ 22. rubidium sulfate _______________________________ 23. aluminum chloride _______________________________ 24. hafnium bromide _______________________________ 25. pandemonium iodide (very rare….) _______________________________