Minilab 5-03 - Solubility Rules - Mini
advertisement

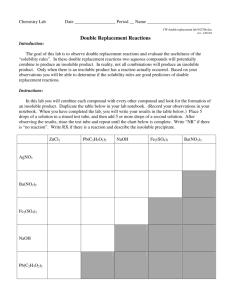
Mini-Lab 5-03: Solubility Rules! Purpose: Do you remember all the solubility rules? Can you figure them out? FYI The so called “solubility rules” are really more like “suggestions”. There are important exceptions to many of the “rules”. Here are two which have no known exceptions: (1) ionic compounds (”salts”) containing metals from group I of the periodic table are always soluble in water, and (2) all nitrate salts are soluble in water. Safety 1) 2) 3) 4) Don’t eat or drink anything in the lab. Always wear eye protection. Wear protective clothing (lab coats, etc.). Don’t play around – treat the lab with respect. Questions Use small amounts of the provided solutions to determine answers to the following questions: 1) Are there exceptions to the rule that salts containing group VII elements (halogens) are usually soluble in water? If so, which group VII compounds are NOT soluble in water? 2) What other “rules” can you observe from the mixtures you have made? What compounds are usually soluble in water? What compounds are usually not soluble in water? 3) Are there exceptions to those “rules”? If so, what are they? 4) You have done all these experiments using water as the solvent. Would these “rules” still be true if you were to use carbon tetrachloride as the solvent? Why or why not? WAIT! Do not write down an answer to the Final question until your Instructor tells you to. 5) FINAL: Write down all the rules that you observed. Instructor’s Page 5-03: Solubility Rules! Source: USAFA Chemtrails – Qualitative Analysis Concepts: solubility rules and their exceptions, intermolecular forces Materials: 6.0 M HCl, 6.0 M NaOH, 3.0 M NH4OH, 0.010 M KMnO4, 0.10 M Al(NO3)3, 0.10 M Ba(NO3)2, 0.10 M Cu(NO3)2, 0.10 M Fe(NO3)3, 0.10 M FeSO4, 0.10 M Pb(NO3)2, 0.10 M Mn(NO3)2, 0.30 M AgNO3, 0.10 M Na2CO3, 0.10 M NaCl, 0.10 M NaI, 0.10 M NaNO3 Hints: This might be a good Mini-Lab to do as a class project, for the sake of time. You could have different groups working with different anions or cations and combining their results. Or, perhaps use groups of 4 students instead of groups of 2 and then have a prize for the group that discovers all the solubility rules and exceptions. The idea is just for the students to make mixtures and record what happens, particularly what things make solid precipitates. This will require them to do some deductive reasoning, such as: “I mixed hydrochloric acid with lead nitrate and saw a white precipitate form. Since I know that any first-column salts and nitrate salts are soluble, then the precipitate must be lead chloride.” The best way to help them think through this is to ask questions that lead them toward the proper conclusions. For example: “What ions were present in that mixture? Were any of them first-group elements or nitrate? What do you know about those compounds?” Discussion ideas: The role of intermolecular forces and their relative strength in solvation and precipitation processes. Is anything really totally insoluble? Talk about what that means. Mercuric Sulfide (HgS) is one of the least soluble salts around, with a Ksp = 4 x 10-53. This means that you would need 263 liters of water just to get ONE MOLECULE of HgS to dissolve! That’s pretty insoluble, but it isn’t TOTALLY insoluble. The difference between very small and zero is infinite. For More information: ‐ For a refresher on solubility product constants and solubility, consult any general chemistry textbook.