Molecular Models
advertisement

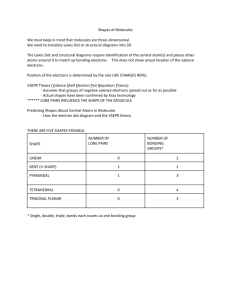
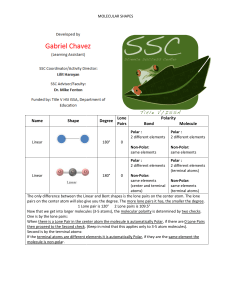
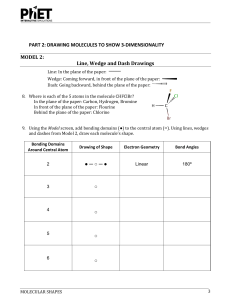
Data and Observations A. Complete the table below. Note that “CA” stands for central atom. Use the element’s symbol to represent the atom. Formula I2 OF2 NI3 SiCl4 BeCl2 BF3 Lewis Dot Diagram (Make sure every atom has the correct # of valence e-) # of Ligands off CA # of Lone Pairs of e- off CA Name of Shape of Molecule 3-D Structural Diagram (indicate lone pairs) Bond Angle Polar or Non-polar molecule? Formula SF6 CH2Cl2 PCl3 HCN PF5 COCl2 Lewis Dot Diagram # of Ligands off CA # of Lone Pairs of e- off CA Name of Shape of Molecule 3-D Structural Diagram (indicate lone pairs) Bond Angle Polar or Non-polar molecule? Name _______________ Chemistry Period ___ Date ________________ Molecular Models LAB Procedure 1. Obtain a molecular model building set. Study the color code identifying the different kinds of atoms. 2. Observe that the following atoms have one hole (bonding site): hydrogen, halogens. The atoms with two holes are oxygen and sulfur. A nitrogen atom has three holes, and carbon and silicon have four holes. 3. Complete column 2, Lewis Dot Diagram, and then construct a model of each molecule listed below. 4. Complete the data table. Analysis and Conclusion 1. Which would repel other electron pairs more: bonding pairs or lone pairs of electrons? Why? _______________________________________________________________________ 2. Name two shapes that always produce polar molecules. Why is this so? _______________________________________________________________________ _______________________________________________________________________ 3. What is meant by the terms “bonding pair of electrons” and “lone pair of electrons”? _______________________________________________________________________ _______________________________________________________________________ 4. Why is it possible for a molecule to have polar bonds, yet be a non-polar molecule? _______________________________________________________________________ _______________________________________________________________________ 5. Define resonance. Give two examples of molecules that show resonance. ________________________________________________________________________ ________________________________________________________________________