Covalent Bonding * POLARITY & INTERMOLECULAR FORCES
advertisement
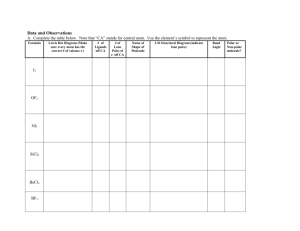
COVALENT BONDING – POLARITY & INTERMOLECULAR FORCES Chem-To-Go Lesson 18 Unit 4 PHYSICAL PROPERTIES The Lewis dot structure of a compound gives you hints about some of the compound’s physical properties. PHYSICAL PROPERTIES Melting: process by which the particles of a substance begin to move more quickly and further apart Boiling: process by which the particles of a substance begin to move EVEN more quickly and EVEN further apart POLARITY OF THE MOLECULE The first step in predicting simple physical properties like melting point and boiling point is to determine the polarity of a molecule. Polar Molecules • Have a lone pair of electrons on the central atom in the Lewis dot structure Nonpolar Molecules • Have no lone pair of electrons on the central atom AND OR • Have one terminal atom that differs from the other terminal atoms (asymmetrical) • All terminal atoms are similar (symmetrical) EXAMPLE #1: CH 4 • Does the molecule have a lone pair of electrons on the central atom? OR • Have one terminal atom that differs from the other terminal atoms? (asymmetrical) EXAMPLE #2: NH 3 • Does the molecule have a lone pair of electrons on the central atom? OR • Have one terminal atom that differs from the other terminal atoms? (asymmetrical) INTERMOLECULAR FORCES Water molecules INTER = BETWEEN • Polar molecule due to a lone pair of electrons on the central O atom • Since O has more electrons surrounding it, the O end of the bond is partially negative. • This leaves the H end of the bond partially positive. • Opposites attract: The partially positive H end of one molecule is attracted to the partially negative O end of another molecule. T YPES OF INTERMOLECULAR FORCES 1) London dispersion forces (LDF) – • • • • Weakest Temporary Between nonpolar molecules Result in low melting & boiling points (easy to melt & boil) 2) Dipole-dipole forces – • Forces of attraction/repulsion that exist between POLAR molecules as a result of the partial charges • Result in high melting & boiling points (difficult to melt & boil) 3) Hydrogen bonding – SPECIAL T YPE OF DIPOLE FORCE • STRONGEST intermolecular force • Occurs when H is bonded to FON creating REALLY strong partial charges • Results in highest melting & boiling points (most difficult to melt & boil) EXAMPLE #3: Br 2 Is the molecule polar? London dispersion forces • Nonpolar only Dipole-dipole forces • Polar only Hydrogen bonding • Polar AND H bonded to F, O, or N EXAMPLE #4: HCl Is the molecule polar? London dispersion forces • Nonpolar only Dipole-dipole forces • Polar only Hydrogen bonding • Polar AND H bonded to F, O, or N EXAMPLE #5: CO 2 Is the molecule polar? London dispersion forces • Nonpolar only Dipole-dipole forces • Polar only Hydrogen bonding • Polar AND H bonded to F, O, or N EXAMPLE #6: H 2O Is the molecule polar? London dispersion forces • Nonpolar only Dipole-dipole forces • Polar only Hydrogen bonding • Polar AND H bonded to F, O, or N