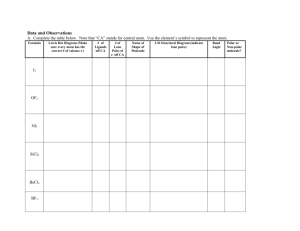
Name Shape Degree Lone Pairs Polarity Bond Molecule Polarity d
advertisement

MOLECULAR SHAPES Name Linear Linear Shape Degree 180° 180° Lone Pairs Polarity Bond Molecule Polar : 2 different elements Polar : 2 different elements Non-Polar: same elements Non-Polar: Non same elements Polar : 2 different elements Polar : 2 different elements (terminal atoms) 0 0 Non-Polar: same elements Non-Polar: Non (center and terminal same elements atoms) (terminal atoms) The only difference between the Linear and Bent shapes is the lone pairs on the center atom. The lone pairs on the center atom will also give you the degree. The more lone pairs it has, the smaller the degree. degree 1 Lone pair is 120 120° 2 Lone pairs is 109.5° Now that we get into larger molecules (3-5 atoms), the molecular polarity is determined by two checks. One is by the lone pairs: When there is a Lone Pair in the center atom the molecule is automatically Polar,, if there are 0 Lone Pairs then proceed to the Second check. (Keep in mind that this applies only to 3-5 5 atom molecules). Second is by the terminal atoms: If the terminal atoms are different elements it is automatically Polar Polar, if they are the same element the molecule is non-polar. MOLECULAR SHAPES Bent 120° 1 Polar : 2 different elements (center and terminal atoms) Most of the bonds will be polar since they will be composed of different elements. Bent 109.5° 2 Polar : 2 Check Process. Check 1: If there is a Lone Pair in the center atom, the molecule is automatically Polar. If there are 0 Lone Pairs then proceed to the Second check. Check 2: If the terminal atoms are different elements. Trigonal Planar 120° 0 Trigonal Pyramidal 109.5° 1 Tetrahedral 109.5° 0 Non-Polar: This is only possible when the center atom has 0 Lone Pairs and the terminal atoms are the same kind of element. MOLECULAR SHAPES The best ways to remember all this information is by breaking everything down into individual parts. 1st remember the names: The names can be determined by the shape and angle of the molecule. Linear = is just a line of atoms with a 180° angle. Notice that it's 2 or 3 atoms total. Bent = Linear but bent due to the Lone Pairs that it contains, the more Lone Pairs the greater the bent and the smaller the degree. It is 3 atoms total. Trigonal Planar = the way I remember this name is by the "Tri" which makes me think of 3 terminal atoms and the word planar makes me think of something plane which means nothing extra (0 Lone Pair). Trigonal Pyramidal = also focus on the “Tri” (3 terminal atoms), but mainly that it contains 1 Lone Pair. Tetrahedral = is a fully bonded molecule, and I use the word "Tetra" to remind me that there are 4 terminal atoms. 2nd: The degrees, notice that when you have the center atom fully loaded on all 4 directions (either by bonds or Lone Pairs) the degree is smaller. So the degrees are totally dependent on the bonds and Lone Pairs. Finally: The Polarity. If you follow the Notes I wrote, you will not have a problem. Good Luck!!