Ch. 8 Molecular Shape
advertisement

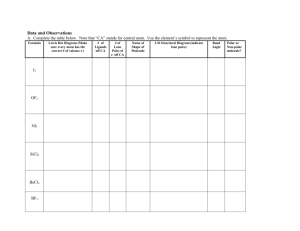
Ch. 8 Molecular Shapes MOLECULAR GEOMETRY aka MOLECULAR GEOMETRY VSEPR • Valence Shell Electron Pair Repulsion theory. • Most important factor in determining geometry is relative repulsion between electron pairs. Molecules adopt the shape that minimizes the electron pair repulsions. Electron clouds of Lone Pairs take up much more space than electron clouds of Shared Pairs and, so repel with more force. (Shared pairs of electrons are trapped in a rather restricted cloud between the nuclei of the atoms sharing them – they can’t spread out much) Predicting a VSEPR Structure Draw Lewis structure. Put electron pairs as far apart as possible around atoms.(Shared pairs must stay together!) Determine positions of atoms from the way electron pairs are shared. Determine the name of geometric structure (shape!) from #’s of shared & unshared pairs of e-’s Structure Determination by VSEPR If there are only 2 atoms in the molecule: Diatomic – 2 like atoms Binary - 2 different atoms Can only be Linear in shape Structure Determination by VSEPR If there are only 3 atoms in the molecule: Two shapes are possible (based on #’s of shared & lone electron pairs around central atom!) Linear & Angular (bent) If: 2 shared pairs & no lone pairs (around central atom!) • With three atoms the farthest they can get apart is 180º. • Shape called Linear. Ex. CO2 180º O C O .O . C . O. If: 2 shared pairs & 1 or 2 lone pairs (around central atom!): Ex. Water, H2O 104.5° •• H O H •• 2 shared pairs 2 lone pairs The molecular geometry is BENT or ANGULAR. Common Molecular Shapes 2 bonding pairs 1 (or 2) lone pairs SO2 BENT or Angular <120° If: 3 atoms attached to a central atom: 2 shapes are possible: Trigonal Planar (3 shared, 0 unshared) Trigonal Pyramidal (3 shared, 1 unshared) If: 3 shared pairs & no lone pair the bonds are all identical, so push apart with equal force (equidistant apart) • The farthest you can the electron pairs apart is 120º. H H C O H H C 120º O •Shape is flat and called TRIGONAL PLANAR. Common Molecular Shapes 3 bonding 0 lone BF3 B TRIGONAL PLANAR A B B 120° If: 3 shared pairs & 1 unshared pair around central atom: One lone pair 3 shared pairs Ammonia, NH3 H N H H H N H H <109.5º The MOLECULAR GEOMETRY — the positions of the atoms — is TRIGONAL PYRAMIDAL. The lone pair’s electron cloud takes up more space then the shared pairs’ clouds 3 bonding 1 lone NH3 TRIGONAL PYRAMIDAL 107° Examples • PF3 3 shared pairs 1 lone pair F P F F TRIGONAL PYRAMIDAL 107° If: 4 atoms around a central atom: 4 shared pairs and no lone pair (all bonds the same – so equidistant apart) • Basic shape is Tetrahedral. • A pyramid with a 109.5º triangular base. • Same basic shape for everything with 4 pairs. H H Ex. CH4 C H H B 4 bonds 0 lone pair A B B B CH4 TETRAHEDRAL 109.5° Methane building blocks CH4 Bonding and Shape of Molecules Number of Bonds Number of Unshared Pairs 0 3 0 4 0 3 1 2 2 Shape Examples -Be- Linear BeCl2 Trigonal planar BF3 Tetrahedral CH4, SiCl4 Pyramidal NH3, PCl3 Angular (Bent) H2O, H2S, SCl2 B C : 2 Covalent Structure : N O: Polar Molecules (Not Polar Bonds!) (We already did those!) Molecules with slightly charged ends! Polar Molecules (Dipoles) • Molecules with a partially positive end and a partially negative end • Requires two things to be true The molecule must contain polar bonds (This can be determined from differences in electronegativity.) Symmetry cannot cancel out the effects of the polar bonds. So….Must determine geometry first. Polarity 1. Draw structural formula in correct shape; 2. Add partial charges (based on electronegativity difference); 3. Can you divide it into a + half & a – half? + - H F + Polar Molecules • Symmetrical shapes are those without lone pair on central atom – Tetrahedral – Trigonal planar – Linear • Will be nonpolar if all the atoms (around central atom) are the same • Shapes with lone pair on central atom are not symmetrical • Will be polar Determining Molecular Polarity • Polar molecules have... – asymmetrical shape (lone pairs) or – asymmetrical atoms + CHCl3 Cl - Shows direction electrons are attracted to H Cl Cl Yes! So molecule is Polar Molecular Polarity • Can you draw a straight line through this molecule & get a + & a – side? Yes! So molecule is Polar Nope! SO molecule is non-polar even though bonds are polar! Nonpolar Covalent Molecules • Equal distribution of charge around a central atom. • Molecule has a symmetrical shape Polar Molecules .. F N O Cl H H Polar H F Polar H B Polar Cl F F Cl Polar F H C Xe F Cl F Cl F H F Nonpolar H F Nonpolar C Cl Cl Nonpolar H H Polar Diatomic molecules - Only 100% pure Nonpolar Covalent bonds• Diatomic molecules include:H2, N2, O2, F2, Cl2, Br2, I2 • These seven elements ALWAYS exist as diatomic molecules if they are: – NOT part of a compound AND – NOT an ion. BrINClHOF or HON 7 3. Helpful hints and practice: A. Hints to help you decide if a molecule is POLAR: 1. Does it have at least one polar bond If so, it's probably polar. 2. Does it have any unshared pair of electron around the central atom? If so, it is probably polar. 3. Can the molecule act like a magnet? If so, it is probably polar. 8.4 Polar Molecules • A hydrogen chloride molecule is a dipole. - + + - H-F Molecular Polarity • This is why oil and water will not mix! Oil is nonpolar, and water is polar. • The two will repel each other, and so you can not dissolve one in the other Molecular Polarity • “Like Dissolves Like” –Polar dissolves Polar –Nonpolar dissolves Nonpolar