Bio H2O Objectives
advertisement

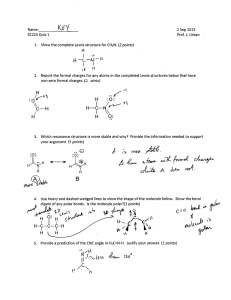
Bio H2O Objectives After reading this section (2.2) and attending class, you should be able to: Describe how water contributes to the fitness of the environment's ability to support life Describe the structure, geometry and polar nature of a water molecule and explain what properties emerge as a result of these characteristics Explain the relationship between the polar nature of water and its ability to form hydrogen bonds List five characteristics of water that result from hydrogen bonding Explain how water's high specific heat, high heat of vaporization and expansion upon freezing affect both aquatic and terrestrial ecosystems Explain how the polarity of the water molecule makes it a versatile solvent and be able to identify those kinds of molecules likely to be highly soluble in water Write the equation for the dissociation of water and explain what is actually transferred from one molecule to another Explain the pH scale Explain how acids or bases directly or indirectly affect the hydrogen ion concentration of a solution