Kinetics: Concept Tests
advertisement

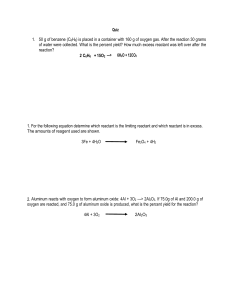
Chapter 15 Concept Tests: Concept Test: The stoichiometry for a given reaction can be used to calculate the rate for any reactant or product in the chemical reaction if another rate is known. 3NO2 (g) + H2O (l) → 2HNO3 (l) + NO (g) the nitrogen dioxide is consumed at a rate of 0.30 mol/Lsec. Express the reaction rate in terms of the concentration of nitric acid 1 Concept Test: List the individual reaction order and overall reaction order for the following reactions: NO(g) + O3 (g) → NO2 (g) + O2 (g) rate = 2NO (g) + 2H2 (g) → N2 (g) + 2H2O (g) rate = (CH)3CBr (l) + H2O (l) → (CH3)3COH (l) + H+1 (aq) + Br-1 (aq) rate = Concept Test: Determine the exponent for each of the following: (2)x = 1 (2)x = 8 (2)x = 2 (2)x = 4 O2 (g) + 2NO (g) → 2NO2 (g) 2 Remember, you want to examine ONE variable at a time. That means that you need to make sure that ALL other variables are held constant. In this case, there are two variables to consider, the concentration of O2 and the concentration of NO. In order to determine the order of each reactant, we need to examine what happens to the rate when one reactant is held constant and the other changes. In experiment 1 and experiment 2, the concentration of NO is held constant while the concentration of O2 increases from 1.10 x 10-2 to 2.20 x 10-2 (the concentration is doubled) What happens to the rate from reaction 1 to reaction 2? The rate increases from 3.21 x 10 -3 to 6.40 x 10-3. The rate also almost doubles (a ratio of 1.994!) So here is the problem set-up: Rate 2 = 2 Rate 1 Concentration Expt 2 = 2 Concentration Expt 1 Remember, the order is related to the concentration raised to some power And that concentration and the power are related to the rate [Concentration Ratio of Variable Changed]x = rate ratio (2)x = 2 x=1 therefore the reaction is first order with respect to O2 In experiment 2 and experiment 4 the concentration of NO is also held constant. The concentration ratio for O2 = 1.5 and the rate ratio =1.5 Therefore, we would calculate the same reactant order if we used those experiments instead. 3 In experiment 1 and experiment 4 the concentration of NO is held constant. The concentration ratio for O2 = 3 and the rate ratio = 3 also. So again, we would get first order for O2. The only “trick” is to make sure that when you determine the ratio for the concentrations you use the same method to determine the rate ratio. Meaning, if you determine the concentration ratio using experiment 4/experiment 1 concentrations, then you MUST calculate the rate ratio using the rate of experiment 4/rate of experiment 1 (and not the reverse). Concept Test: Using the above data, determine the reactant order for NO Write the rate law Determine the overall reaction order However, just because the exponents work out to be nice numbers, does not mean that the rate ratios or concentration ratios will. The following is a mathematical property of logarithms, and it should be used when determining the exponent might not be so intuitive: log Ab = b x log A Just a helpful math trick to help you determine the exponent: HINT: this may be useful for lab!! For example, (3.77)x = 53.58 x (log 3.77) = log 53.58 x = log 53.58 log 3.77 x = 1.729 4 0.5763 x = 3.000 NOTE!!!: log 53.58 log 3.77 does NOT equal 5 log 53.98 3.77