File
advertisement
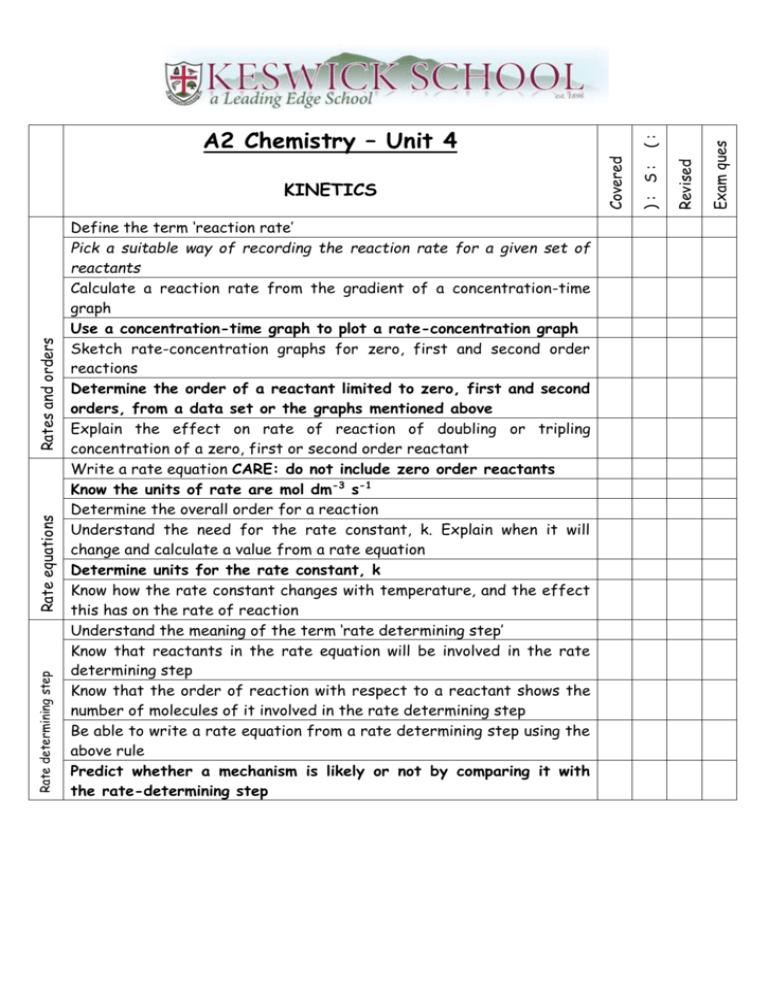
Rate determining step Rate equations Rates and orders Define the term ‘reaction rate’ Pick a suitable way of recording the reaction rate for a given set of reactants Calculate a reaction rate from the gradient of a concentration-time graph Use a concentration-time graph to plot a rate-concentration graph Sketch rate-concentration graphs for zero, first and second order reactions Determine the order of a reactant limited to zero, first and second orders, from a data set or the graphs mentioned above Explain the effect on rate of reaction of doubling or tripling concentration of a zero, first or second order reactant Write a rate equation CARE: do not include zero order reactants Know the units of rate are mol dm-3 s-1 Determine the overall order for a reaction Understand the need for the rate constant, k. Explain when it will change and calculate a value from a rate equation Determine units for the rate constant, k Know how the rate constant changes with temperature, and the effect this has on the rate of reaction Understand the meaning of the term ‘rate determining step’ Know that reactants in the rate equation will be involved in the rate determining step Know that the order of reaction with respect to a reactant shows the number of molecules of it involved in the rate determining step Be able to write a rate equation from a rate determining step using the above rule Predict whether a mechanism is likely or not by comparing it with the rate-determining step Exam ques Revised ): S: Covered KINETICS (: A2 Chemistry – Unit 4