Rate Law Notes sheet
advertisement

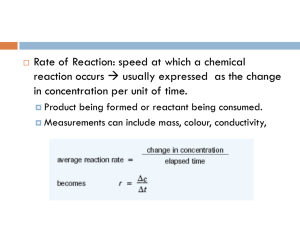
Rate Law Rate Law – used to show the effects of reactant concentrations on rate of chemical reactions Rate = k[A]x[B]y[C]z generic form Variables: Rate – average rate of reaction k – rate constant [A], [B], [C] – concentration (M) of reactants x, y, z – usually small whole numbers that are calculated By analyzing data from different experiments where the concentration of one of the reactants is changed while the others are held constant, we can determine whether the change will have an effect on the rate of the reaction. Using the information from the experiments, we can calculate the values of x, y, z, and k to produce our usuable Rate Law. THERE IS NO WAY TO THEORETICALLY FIND THE VALUES OF x, y, and z!!! YOU MUST USE EXPERIMENTAL DATA TO CALCULATE!! An example of the process: Determine the rate law for the following reaction using the following data: Na2CO3 + CaF2 CaCO3 + 2NaF Experiment # [Na2CO3] 1 2 3 0.200 M 0.400 M 0.400 M [CaF2] 0.200 M 0.200 M 0.400 M Initial Rate of Reaction 2.2 x 10-3 M/s 4.4 x 10-3 M/s 8.8 x 10-3 M/s Step #1: Start by writing out the generic rate law including the actual reactants Step #2: Now we must find the value of x a) Since we are looking for the value of x and x is the exponent on Na2CO3, we need to find two experiments where the concentration of Na2CO3 was changed and the other concentration(s) was held constant. b) Write a separate rate law equation for each experiment plugging in the concentration and rates from the table: c) Write a ratio of the two rate laws (always put the one with the larger rate on the top) and solve for x: Step #3: Now we must find the value of y a) Since we are looking for the value of y and y is the exponent on CaF2, we need to find two experiments where the concentration of CaF2 was changed and the other concentration(s) was held constant. b) Write a separate rate law equation for each experiment plugging in the concentration and rates from the table: c) Write a ratio of the two rate laws (always put the one with the larger rate on the top) and solve for y: Step #4: Rewrite the rate law with the exponents written in What do the exponents mean???? If the exponent is zero, then that reactant’s concentration has no effect on the rate of the reaction (this is referred to as a ZERO ORDER) If the exponent is one, then doubling that reactant’s concentration with double the rate, tripling the concentration of the reactant will triple the rate, etc. (this is referred to as FIRST ORDER) If the exponent is two, then doubling the reactant’s concentration will increase the rate by four times (22 = 4), tripling the reactant’s concentration will increase the rate by nine times (32 = 9), etc. (this is referred to as SECOND ORDER) Step #5: Calculate the value of k a) Use the data from ANY one of the experiments and plug it into the rate law you developed in Step #4 and solve for k. b) Determine the unit on k Step #6: Rewrite the rate law with the values for the exponents and for k plugged in Now you can use this rate law to determine the rate of reaction for any combination of concentrations for the reactants!! This the point of determining a reaction's Rate Law. Practice Problem: Determine the rate law for the reaction, A + B C + D using the data. Experiment # [A] [B] Initial Rate of Reaction 1 2 3 0.100 M 0.100 M 0.400 M 0.200 M 0.600 M 0.600 M 1.1 x 10-6 M/s 9.9 x 10-6 M/s 9.9 x 10-6 M/s