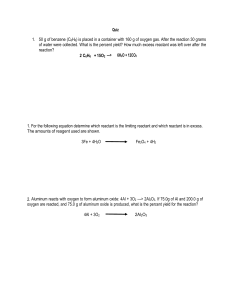
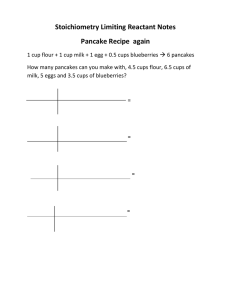
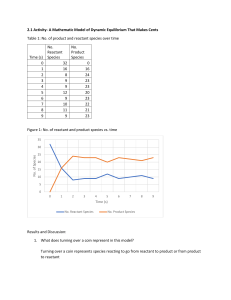
The reaction between solid white phosphorus (P4) and oxygen
advertisement

3rd hour Wednesday, March 05, 2014 9:43 AM Pre-lab discussion: The reaction between solid white phosphorus (P4) and oxygen produces solid tetraphosphorus decoxide. Write the balanced equation for this reaction. Determine the mass of tetraphosphorus decoxide formed if 25.0 g P4 and 50.0 g oxygen are combined. What is the limiting reactant? What mass of the excess reactant remains (unused) after the reaction stops? LimRxt2 Page 1 LimRxt2 Page 2 4th hour Wednesday, March 05, 2014 9:43 AM The reaction between solid sodium and iron (III) oxide is one in a series of reactions that inflates an automobile airbag: ___ Na (s) + ___ Fe2O3 (s) → ___ Na2O (s) + ___ Fe (s) If 100.0 g of Na and 100.0 g of Fe2O3 are used in the reaction, determine the following: a. Limiting reactant? a. Excess reactant? a. Mass of solid iron produced? a. Mass of excess reactant used? a. Mass of excess reactant remaining (unused)? LimRxt2 Page 3 Actual Yield: Percent Yield: LimRxt2 Page 4