REDOX Half Reaction Method in Acidic Solutions -- STEPS
advertisement

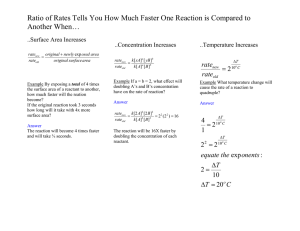
REDOX Half Reaction Method in Acidic Solutions -- STEPS 1) Using the given partial net ionic equation, write two ½ reactions. Note that one is a reduction and one is an oxidation. Also, all species present in the given equation must be included in one of the half reactions. 2) Time to do some balancing. For each of the half reactions: A) Balance all elements except H and O, by adding coefficients as necessary. B) Balance O by adding H2O to either the reactant side or product side. C) Balance H by adding H+ to either the reactant side or the product side. D) Balance charge by adding e- to either the reactant side or the product side. 3) Multiply each ½ reaction by some whole number such that e- gained = e- lost. 4) Add ½ reactions together to create one large equation. 5) Simplify this equation by cancelling like expressions. 6) Check to make sure atoms and charge are balanced. DONE