Section 2A: Solubility, Ksp, and Complex Ions
advertisement

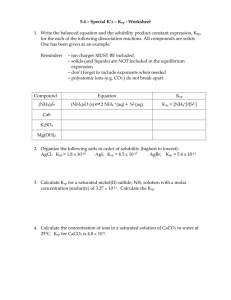
Section 2A: Solubility, Ksp, and Complex Ions......................Jenni Morris and Aaron Elinoff •Solubility is the quantity of a particular substance that can dissolve in a particular solvent (yielding a saturated solution) Henry's Law: Sg = kHPg •Sg = gas solubility (M) •kH = Henry’s law constant (M/mmHg) •Pg = partial pressure of the gaseous solute (mmHg) Le Chatelier's Principle: Gas + liquid solvent <----> saturated solution + heat energy •A change in any of the factors determining equilibrium causes the system to shift.... In this case, the reaction shifts left •For the Reaction: AaBb(s) <----> aAb+(aq) + bBa+(aq) –The solubility expression is: Ksp = [Ab+]a[Ba-]b and Q = [Ab+]a[Ba-]b •What’s the difference between Q and Ksp? –Q is used for a chemical reaction that is not necessarily at eq. –Q = Ksp, the system is at equilibrium and is saturated –Q < Ksp, the system is not at eq. and is not saturated –Q > Ksp, the system is not at eq. and is supersaturated The Common Ion effect •Using: Ksp = [Ag+][Cl-] = 1.6x10-10 •We know: (x)(x) = 1.6x10-10 •And: x = [Ag+] = [Cl-] = 1.3 x 10 -5 •So what if we added 0.10 mol of NaCl to 1 liter of AgCl solution? •[Ag+][Cl-] = 1.6x10-10 [Ag+](0.10M) = 1.6x10-10 [Ag+] = 1.6x10-9 Complex Ions •A complex ion is formed by combination of simpler ions or molecules –Example: Co2+ combines with 6 H2O to form the complex ion Co(H2O)62+